Abstract
Background
Soluble sugars are involved in responses to stress, and act as signalling molecules that activate specific or hormone cross-talk transduction pathways. Thus, exogenous sucrose treatment efficiently induces tolerance to the herbicide atrazine in Arabidopsis thaliana plantlets, at least partially through large-scale modifications of expression of stress-related genes.
Methods
Availability of sugars in planta for stress responses is likely to depend on complex dynamics of soluble sugar accumulation, sucrose–starch partition and organ allocation. The question of potential relationships between endogenous sugar levels and stress responses to atrazine treatment was investigated through analysis of natural genetic accessions of A. thaliana. Parallel quantitative and statistical analysis of biochemical parameters and of stress-sensitive physiological traits was carried out on a set of 11 accessions.
Key Results
Important natural variation was found between accessions of A. thaliana in pre-stress shoot endogenous sugar levels and responses of plantlets to subsequent atrazine stress. Moreover, consistent trends and statistically significant correlations were detected between specific endogenous sugar parameters, such as the pre-stress end of day sucrose level in shoots, and physiological markers of atrazine tolerance.
Conclusions
These significant relationships between endogenous carbohydrate metabolism and stress response therefore point to an important integration of carbon nutritional status and induction of stress tolerance in plants. The specific correlation between pre-stress sucrose level and greater atrazine tolerance may reflect adaptive mechanisms that link sucrose accumulation, photosynthesis-related stress and sucrose induction of stress defences.
Key words: Arabidopsis thaliana, natural variation, atrazine sensitivity, carbon nutrition, endogenous soluble sugars, oxidative stress, sucrose, xenobiotic stress
INTRODUCTION
Glucose (Glc) and fructose (Fru) are ubiquitous sources of carbon, reducing power and energy for eukaryotic cells, and in oxygenic photosynthetic organisms, especially in higher plants, sucrose (Suc) is particularly important for transport, allocation and source–sink utilization of photosynthesis-assimilated carbon (Salerno and Curatti, 2003). These soluble sugars are also involved in signalling processes that result in altered expression of genes related to carbohydrate and nitrogen metabolism, metabolite transport and stress responses (Price et al., 2004; Thum et al., 2004; Loreti et al., 2005; Ramel et al., 2007).
Many environmental stresses lead to major changes in carbohydrate metabolism. Under conditions such as drought, desiccation, salt stress or low temperature, soluble sugars are accumulated at high concentrations, and are thought to act as protective compatible solutes (Korn et al., 2008). Soluble sugars have also been involved in other stress conditions, where they do not accumulate at the same levels as those resulting from drought or freezing stress, and thus do not act as compatible solutes. Thus, pre-treatment with Suc enhances resistance of rice plants to Magnaporthe oryzae infection (Gomez-Ariza et al., 2007) and tolerance of Arabidopsis thaliana plantlets to anoxic stress (Loreti et al., 2005). Exogenous supply of soluble sugars has also been shown to confer to Arabidopsis plantlets tolerance to atrazine-mediated xenobiotic and oxidative stress (Sulmon et al., 2004, 2006, 2007). As in the case of induction of tolerance to anoxic stress (Loreti et al., 2005), the protective effects of sugar treatment could not be ascribed to mere carbon feeding of pre-existing pathways. Induction of tolerance was associated with important modifications of gene expression related to reactive oxygen species (ROS) defence and repair mechanisms and to metabolic re-orientation, and seemed to result from complex interactions involving sugar and xenobiotic signalling (Ramel et al., 2007).
Characterization of the involvement of soluble sugars in stress responses is often based on exogenous treatments (Loreti et al., 2005; Ramel et al., 2007). However, exogenous treatments may differ from endogenous variations, both in quantitative terms and in terms of tissue partitioning and metabolic targeting. Endogenous variations of soluble sugars have thus been studied in Arabidopsis mutants affected in genes involved in sucrose/starch partitioning, such as the starchless pgm mutant (Gibon et al., 2004). However, mutations in key genes of starch synthesis may result in an increase in soluble sugar in compartments and metabolic pathways that differ from those that may be modified by natural selection. In contrast, genetic and trait variation among A. thaliana wild-type lines (Koornneef et al., 2004) provides an excellent tool to examine natural endogenous fluctuations and tolerance variations, and to gain insight into physiological, metabolic and regulatory networks in an ecological and evolutionary context (Cross et al., 2006). Previous studies of Arabidopsis accessions have revealed contrasted metabolic phenotypes, including important variations in carbohydrate levels and night–day fluctuations (Cross et al., 2006; Davey et al., 2008). Moreover, Korn et al. (2008) have shown significant correlations between leaf freezing tolerance and endogenous overaccumulation of Glc, Fruc, Suc and raffinose, which are part of the response to freezing stress.
Since our previous work (Ramel et al., 2007, 2009) had shown that interactions between Suc and atrazine were essential for induction of stress defences, we aimed to investigate the impact of endogenous carbon nutritional status on the efficiency of stress response induction. We thus used a natural variation approach in order to analyse relationships between pre-stress endogenous sugar variations and differential responses to xenobiotic stress involving photosynthesis inhibition and oxidative stress. Quantitative analysis of biochemical traits and physiological responses revealed that pre-stress absolute sugar levels were correlated with subsequent xenobiotic stress tolerance. Among the different sugar traits, the absolute level of Suc at the end of the day was found to be particularly important for responses of stress tolerance. This specific importance of Suc is discussed in relation to the nature of atrazine-mediated stress, which is light and photosynthesis dependent.
MATERIALS AND METHODS
Arabidopsis accessions
Arabidopsis thaliana accessions used in this study, Col-0, Bch-1, Bur-0, Eil-0, Lip-0, Mt-0, Pla-0, Ta-0, Te-0, Rsch-0 and Ws (Table 1), were obtained from the Nottingham Arabidopsis Stock Centre (NASC). Accessions were homogenized by single-seed propagation and were bulk amplified prior to the analysis.
Table 1.
Arabidopsis thaliana accessions used in the experiments
| Accessions | NASC no. | Geographical origin | Altitude (m) |
|---|---|---|---|
| Bch-1 | N957 | Bachen (Germany) | 1–100 |
| Bur-0 | N1029 | Burren (Ireland) | Not given |
| Col-0 | – | Landsberg (Poland) | 100 |
| Eil-0 | N1133 | Eilenburg (Germany) | 100–200 |
| Lip-0 | N1337 | Lipowiec (Poland) | 500 |
| Mt-0 | N1381 | Martuba (Libya) | 100–200 |
| Pla-0 | N1459 | Playa de Aro (Spain) | 0–100 |
| Ta-0 | N1549 | Tabor (Czechoslovakia) | 400–500 |
| Te-0 | N1551 | Tenela (Finland) | 1–100 |
| Rsch-0 | N1491 | Rschew (Russia) | 100–200 |
| Ws | – | Wassilewskija (Russia) | 100–200 |
Accessions were obtained from the Nottingham Arabidopsis Stock Centre (NASC).
Plant material and growth conditions
Seeds of A. thaliana were surfaced-sterilized in bayrochlore/ethanol (1 : 1, v/v), rinsed in absolute ethanol and dried overnight. Germination and growth were carried out under axenic conditions in square Petri dishes. After seeds were sown, Petri dishes were placed at 4 °C for 48 h in order to break dormancy and homogenize germination, and transferred to a control growth chamber at 22 °C under a 16 h light period regime at 85 µmol m−2 s−1. Growth medium consisted of 0·8 % (w/v) agar in 1× Murashige and Skoog (MS) basal salt mix (M5519, Sigma-Aldrich) adjusted to pH 5·7. Direct exposure to atrazine during germination and early growth was carried out for 1 week in the absence or presence of 0·25, 0·5 or 1 µm atrazine. Transfer experiments were also carried out in order to compare the effects of atrazine on plantlets at the same stage of development. In these experiments, plantlets were grown for 2 weeks on control MS medium until stage 1·04 of development (as described by Boyes et al., 2001), and then tranferred for 5 d to fresh medium in the absence or presence of 10 µm atrazine. In the presence of this concentration of atrazine, the development of lethal injuries in Arabidopsis plantlets followed a time-dependent pattern over this period.
Analysis of plantlet growth and of photosynthetic parameters
The length of the primary root was measured every day on 30 plantlets grown on vertical plates, and the experiment was repeated at least twice. Results were given as the mean (±s.e.m.) of these determinations. Pigments were extracted by pounding shoots of plantlets in 80 % acetone, and absorbance of the resulting extracts was measured at three wavelengths: 663, 646 and 470 nm. Levels of chlorophylls and total carotenoids (xanthophylls and carotenes) in these extracts were determined from the equations given by Lichtenthaler and Wellburn (1983). Measurements were done on three replicates of 5–10 pooled plantlets each per experiment. Results were given as the mean (±s.e.m.) of these determinations from at least two independent experiments. Chlorophyll fluorescence and maximum photosystem II (PSII) efficiency (Fv/Fm) were measured with a PAM-210 chlorophyll fluorometer system (Heinz Walz, Effeltrich, Germany). After dark adaptation for at least 30 min, minimum fluorescence (F0) was determined under weak red light. Maximum fluorescence of dark-adapted leaves (Fm) was measured under a subsequent saturating pulse of red light, and variable fluorescence (Fv = Fm − F0) was determined. Measurements were carried out on at least 30 plants, and the experiment was repeated at least twice.
Analysis of soluble sugars
For each accession, analysis of soluble sugars was carried out on pools of four independent samples of shoots from 2-week-old plantlets. Sampling was performed in the last hour of the day (end of day, EoD) or of the night (end of night, EoN). Experiments for sugar analysis were repeated at least twice. Samples were ground to powder in liquid nitrogen and extracted in 80 % ethanol containing 4 mm HEPES-KOH, pH 7·5, at 80 °C for 30 min. Samples were then centrifuged for 15 min at 11 000 g. This initial supernatant was collected and stored on ice. The pellet was resuspended in 80 % ethanol containing 4 mm HEPES-KOH (pH 7·5), and incubated at 80 °C for 30 min. After centrifugation of the extract, for 15 min at 11 000 g, the supernatant was collected and stored on ice. This hot extraction of the remaining pellets was repeated further, once with 50 % ethanol in 4 mm HEPES-KOH (pH 7·5) and once with 4 mm HEPES, pH 7·5. All of the resulting supernatants were then pooled and assayed for soluble sugars (Strand et al., 1999). Quantification was carried out spectrophotometrically by enzyme-based assays using the ENZYPLUS® EZS 864+ (RAISIO) kit.
Analysis of ROS
The nitroblue tetrazolium (NBT; N6876, Sigma-Aldrich) staining method of Rao and Davis (1999) was modified for in situ detection of the superoxide radical. Two-week-old plantlets were transferred for 48 and 72 h to fresh medium in the absence or presence of 10 µm atrazine. At the end of the treatment, plantlets were immersed and infiltrated under vacuum with 3·5 mg mL−1 NBT staining solution in potassium phosphate buffer (10 mm) containing 10 mm NaN3. After infiltration, stained plantlets were bleached in acetic acid : glycerol : ethanol (1 : 1 : 3) (v/v/v) solution at 100 °C during 5 min. Plantlets were then stored in a glycerol : ethanol (1 : 4) (v/v) solution until photographs were taken. O2·− was visualized as a blue colour produced by NBT reduction to formazan. Experiments were repeated four times, and each was carried out on at least 15 plantlets.
The H2O2 staining agent, 3,3′diaminobenzidine (DAB; D5637, Sigma-Aldrich), was dissolved in H2O and adjusted to pH 3·8 with KOH. The DAB solution was freshly prepared in order to avoid auto-oxidation (Fryer et al., 2002). Two-week-old plantlets were transferred for 48 and 72 h to fresh medium in the absence or presence of 10 µm atrazine. At the end of the treatment, plantlets were immersed and infiltrated under vacuum with 1·25 mg mL−1 DAB staining solution. Stained plantlets were then bleached in acetic acid : glycerol : ethanol (1 : 1 : 3, v/v/v) solution at 100 °C during 5 min, and then stored in glycerol : ethanol (1 : 4, v/v) solution until photographs were taken. H2O2 was visualized as a brown colour due to DAB polymerization. Experiments were repeated four times, and each was carried out on at least 15 plantlets. The specificity of DAB staining towards H2O2 was assessed in control infiltrations in the presence of 10 mm ascorbic acid.
Statistical analysis
Statistical analysis was carried out with the STATISTICA version 7 software and the Minitab® 15·1·1·0 software (Minitab SARL, Paris, France). Pairwise comparison of means used the non-parametric Mann–Whitney test or the parametric Student's t-test. Values of significance (P) are given in parentheses. Normality of data was analysed by the Anderson–Darling test. A one-way analysis of variance (ANOVA) associated with a Duncan test was used to compare every pair of means within and between accessions. A two-way ANOVA was used to partition the variance of physiological parameters (Fv/Fm, shoot chlorophyll content, shoot carotenoid content) between genetic and experimental variance with the following mixed model :
where μ is the general mean, Ai is the random effect of the ith accession genotype, Tj is the fixed effect of the jth treatment, Ai × Tj is the interactive effect of the ith accession genotype with the jth treatment and εjik is the error of Yijkm.
A one-way ANOVA was used to partition the variance of biochemical parameters (shoot endogenous soluble sugars) into genetic variance with the following general linear model:
where μ is the general mean, Ai is the random effect of the ith accession genotype and εik is the error of Yikm.
Correlations between all of the values for the biochemical and physiological traits were measured with the Pearson coefficient calculated with Minitab® 15·1·1·0 software (Minitab SARL, Paris, France). In order to examine and visualize relationships between biochemical and physiological traits and to determine to what extent the multivariate phenotypes of accessions were distinct, a principal component analysis (PCA) based on the correlation matrix was calculated using the ADE4 package of R software.
RESULTS
Natural variation of xenobiotic sensitivity during germination and early development in the presence of atrazine
The natural variability of 11 accessions of A. thaliana was used to compare the effects of natural metabolic variations on the sensitivity to xenobiotic stress. Physiological and developmental parameters, such as Fv/Fm, chlorophyll content, carotenoid content and primary root growth, were measured during early development of plantlets germinated and grown on MS–agar medium in the absence or presence of varying concentrations of atrazine.
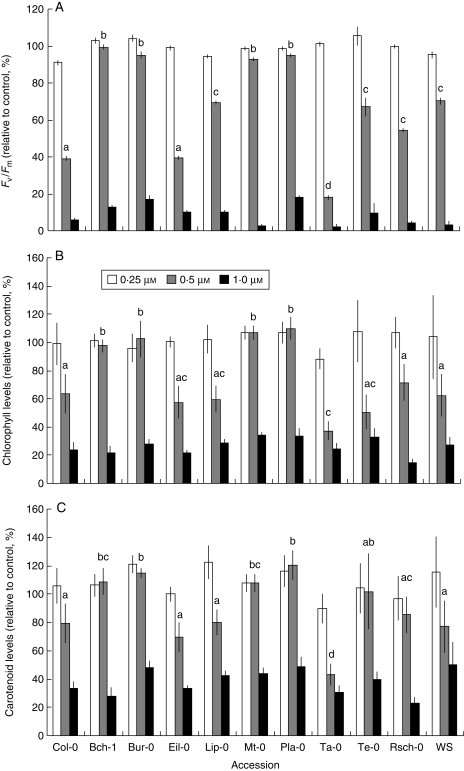
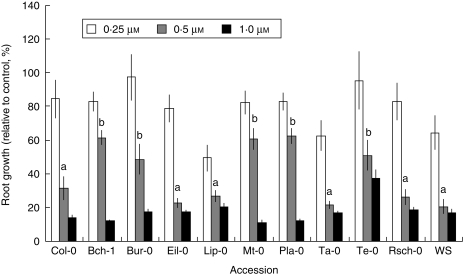
Large variations of all the photosynthetic parameters under study were observed among accessions, with 0·5 µm atrazine treatment giving the most contrasted differences (Fig. 1). Several accessions, such as Bch-1, Bur-0, Mt-0 and Pla-0, maintained significantly higher levels of chlorophyll fluorescence (Fig. 1A) or photosynthetic pigments (Fig. 1B, C), which revealed a lower sensitivity of these genotypes to atrazine treatment. In contrast, the accession Ta-0 presented the lowest values for all the physiological and developmental parameters measured (Fig. 1A–C). The other accessions including Col-0 presented intermediate values. Analysis of primary root growth in the presence of 0·25, 0·5 or 1 µm atrazine (Fig. 2) indicated that natural variation of the response to atrazine also involved differences in growth and development. Treatment with 0·5 µm atrazine gave the most contrasted responses between accessions. The Te-0 accession and the accessions previously showing lower sensitivity to atrazine in terms of photosynthetic parameters (Bch-1, Bur, Mt-0 and Pla-0) presented the highest rates of primary root growth in the presence of 0·5 μm atrazine. All of the other accessions presented greater sensitivity to 0·5 µm atrazine.
Fig. 1.
Effects of atrazine on the photosynthetic apparatus of 1-week-old plantlets of the different accessions germinated and grown in the presence of atrazine. Fv/Fm was determined on whole plantlets (A); chlorophyll (B) and carotenoid (C) levels were measured on shoots. Results (mean ± s.e.m.) are expressed as a percentage relative to growth in the absence of atrazine; treatment concentrations of atrazine are as indicated. Statistical comparison between the means was carried out using one-way ANOVA followed by Duncan's test. Statistical significance of differences (P ≤ 0·05) between accessions subjected to 0·5 µm atrazine is indicated by different letters above bars.
Fig. 2.
Effects of atrazine on primary root growth of 1-week-old plantlets of the different accessions germinated and grown in the presence of atrazine. Results (mean ± s.e.m.) are expressed as a percentage of growth relative to control plantlets grown in the absence of atrazine; treatment concentrations of atrazine are as indicated. Statistical comparison between means was carried out using one-way ANOVA followed by Duncan's test. Statistical significance of differences (P ≤ 0·05) between accessions subjected to 0·5 µm atrazine is indicated by different letters above bars.
Natural variation of xenobiotic sensitivity in Arabidopsis plantlets after transfer to 10 μm atrazine at the same stage of development
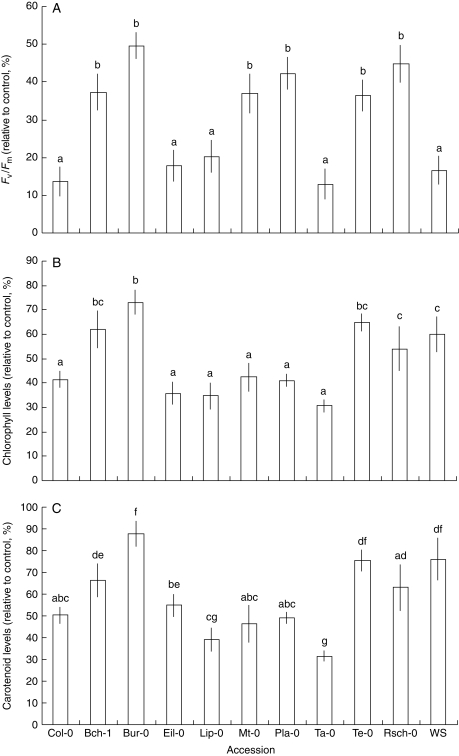
Transfer of 2-week-old plantlets grown on MS control medium onto 10 µm atrazine-containing medium for 5 d was carried out in order to assess the effects of atrazine on plantlets at the same stage of development. Analysis of chlorophyll fluorescence (Fig. 3A) showed two distinct groups of responses among the different accessions. Accessions that showed low or intermediate sensitivity to atrazine during early development (Bch-1, Bur-0, Mt-0, Pla-0, Te-0 and Rsch-0) were also found to be the least sensitive to transfer onto 10 µm atrazine (Fig. 3A). Analysis of chlorophyll and carotenoid levels (Fig. 3B, C) revealed a wider range of responses to atrazine and contrasted results between the transfer experiment and the direct exposure experiment during germination and post-germination (Fig. 1). The Ta-0 accession showed high sensitivity to atrazine, both during transfer onto 10 µm atrazine and during direct exposure to 0·5 µm atrazine. However, in contrast, the Mt-0 and Pla-0 accessions showed high sensitivity to transfer onto 10 µm atrazine, whereas they showed greater tolerance to 0·5 µm atrazine during germination and post-germination (Fig. 1).
Fig. 3.
Effects of atrazine on the photosynthetic apparatus of plantlets of the different accessions grown for 2 weeks in the absence of atrazine and transferred for 5 d to fresh medium in the absence or presence of 10 µm atrazine. Fv/Fm was determined on whole plantlets (A); chlorophyll (B) and carotenoid (C) levels were measured on shoots. Results (mean ± s.e.m.) are expressed as the percentage relative to the values obtained on plantlets of the same genotype transferred for 5 d to control medium in the absence of atrazine. Statistical comparison between means was carried out using one-way ANOVA followed by Duncan's test. Statistical significance of differences (P ≤ 0·05) between accessions is indicated by different letters above bars.
All of this demonstrated differences of responses between experimental modalities and therefore suggested differences of responses in relation to developmental processes. In this transfer experiment, there was no significant difference of primary root growth between the 11 different accessions after 5 d of transfer in the presence of 10 µm atrazine (data not shown).
Natural variation of endogenous soluble sugar levels in Arabidopsis plantlets
The quantification of endogenous soluble sugar contents (Glc, Fru and Suc) at the end of day and at the end of night was carried out on shoots of 2-week-old plantlets for the 11 accessions grown on MS–agar medium. It thus corresponded to the carbohydrate status of plantlets prior to transfer in the absence or presence of 10 µm atrazine.
At the end of day, values of shoot total sugars ranged from 175 ng mg−1 fresh weight (f. wt) for Lip-0 to 754·6 ng mg−1 f. wt for Bur-0 (Table 2). The Lip-0 accession presented the lowest values for Glc, Suc and Fru contents, while the Bur-0 accession showed the highest values for all three soluble sugars. At the end of night, values of shoot total sugars ranged from 198·3 ng mg−1 f. wt for Eil-0 to 438 ng mg−1 f. wt for Bur-0 (Table 3). For all accessions, the pool of Suc was not entirely depleted at the end of night. Thus, at the end of night and at the end of day, the Bur-0 accession presented the highest level of total sugars in the shoots.
Table 2.
Levels of endogenous soluble sugars at the end of day in plantlets of the different accessions
| Accessions | Glc ± s.e. (ng mg−1 f. wt) | Suc (ng mg−1 f. wt) | Fru (ng mg−1 f. wt) | Reducing sugars (ng mg−1 f. wt) | Total sugars (ng mg−1 f. wt) |
|---|---|---|---|---|---|
| Bch-1 | 136·7±22 | 90±10 | 41·3±11 | 178 | 267·9 |
| Bur-0 | 445·7±71 | 236·9±35 | 72·1±16 | 517·8 | 754·6 |
| Col-0 | 128·1±20 | 70·3±9 | 17·5±1 | 145·5 | 215·9 |
| Eil-0 | 262·2±54 | 107·5±17 | 53·9±13 | 316·1 | 423·6 |
| Lip-0 | 104·3±8 | 56·4±9 | 14·2±2 | 118·6 | 175 |
| Mt-0 | 115·6±15 | 78·4±7 | 24·3±5 | 139·9 | 218·3 |
| Pla-0 | 236·1±39 | 211·5±26 | 72·3±17 | 308·4 | 519·9 |
| Rsch-0 | 114·9±14 | 107±11 | 24·1±3 | 139 | 246 |
| Ta-0 | 182·7±16 | 129·4±20 | 44·1±4 | 226·7 | 356·1 |
| Te-0 | 228·1±25 | 79·2±11 | 42·9±5 | 270·9 | 350·2 |
| Ws | 229·4±55 | 138·4±18 | 70·4±21 | 299·8 | 438·1 |
Soluble sugar levels were measured as described in the Methods in shoots of plantlets grown for 2 weeks in the absence of atrazine.
Table 3.
Levels of endogenous soluble sugars at the end of night in plantlets of the different accessions
| Accessions | Glc ± s.e. (ng mg−1 f. wt) | Suc (ng mg−1 f. wt) | Fru (ng mg−1 f. wt) | Reducing sugars (ng mg−1 f. wt) | Total sugars (ng mg−1 f. wt) |
|---|---|---|---|---|---|
| Bch-1 | 131 ± 21 | 86·4 ± 11 | 21·7 ± 3 | 152·7 | 239·2 |
| Bur-0 | 308·3 ± 22 | 94·6 ± 7 | 35·2 ± 2 | 343·4 | 438 |
| Col-0 | 129·7 ± 15 | 101·4 ± 20 | 46·7 ± 7 | 176·4 | 277·8 |
| Eil-0 | 103·3 ± 6 | 76·2 ± 28 | 18·8 ± 4 | 122·1 | 198·3 |
| Lip-0 | 101·5 ± 6 | 99·6 ± 16 | 19 ± 2 | 120·5 | 220·1 |
| Mt-0 | 134·8 ± 10 | 124·7 ± 15 | 31·8 ± 3 | 166·7 | 291·4 |
| Pla-0 | 187·7 ± 7 | 65·9 ± 5 | 66·2 ± 5 | 253·9 | 319·8 |
| Rsch-0 | 175·2 ± 3 | 82·6 ± 6 | 51 ± 3 | 226·1 | 308·7 |
| Ta-0 | 239·7 ± 27 | 33·4 ± 7 | 65·1 ± 8 | 304·8 | 338·1 |
| Te-0 | 229·3 ± 14 | 75 ± 13 | 65·3 ± 4 | 294·5 | 369·5 |
| Ws | 110 ± 5 | 91·4 ± 6 | 54·2 ± 4 | 164·2 | 255·6 |
Soluble sugar levels were measured as described in the Methods in shoots of plantlets grown for 2 weeks in the absence of atrazine.
Important natural variation between accessions was therefore found for all soluble sugars in shoots of plantlets at the end of night and at the end of day (Tables 2 and 3), in general agreement with previous studies on rosettes of mature plants (Cross et al., 2006).
Statistical analysis of environmentally and genetically determined variations
Analysis of potential variations of response to atrazine stress between the 11 accessions of A. thaliana was carried out by a two-way ANOVA on the whole set of absolute individual values of photosynthetic parameters (Fv/Fm, shoot chlorophyll content, shoot carotenoid contents) of the different arabidopsis accessions during transfer of plantlets in the absence or presence of 10 µm atrazine. The F statistics resulting from the ANOVA (Table 4) showed the main effects of genotype and treatment (absence or presence of 10 µm atrazine) and the (genotype × treatment) interaction effect. The ANOVA showed that the three physiological traits were significantly variable, thus showing significant genetic variation among the set of 11 accessions of A. thaliana (FFv/Fm = 11·88, Fchlorophyll = 7·90, Fcarotenoid = 7·25). Table 4 also indicated highly significant effects of atrazine treatment on all three physiological parameters related to photosynthetic activity. In addition, significant (genotype × treatment) interactions were found for the three traits, which indicated interaccession differentiation for plasticity (Table 4).
Table 4.
F statistics of the variations of physiological parameters in plantlets of the different accessions
| Trait | Genotype effect | Treatment effect | Genotype × treatment |
|---|---|---|---|
| Fv/Fm | 11·88*** | 2718·39*** | 8·65*** |
| Chlorophyll content | 7·90*** | 349·42*** | 4·46*** |
| Carotenoid content | 7·25*** | 207·24*** | 5·58*** |
Absolute levels of physiological parameters (Fv/Fm, shoot chlorophyll content and shoot carotenoid content) were measured as described in the Methods in plantlets grown for 2 weeks in the absence of atrazine and transferred for 5 d in the absence or presence of 10 µm atrazine. The whole set of accessions and of data was analysed using a two-way ANOVA with the genotype and treatment as main effects, and with genotype × treatment as interaction effect. *** P < 0·001.
A second analysis, using a one-way ANOVA, was carried out on the whole set of absolute individual values of endogenous soluble sugar levels (Glc, Suc and Fru) at the end of day and at the end of night in shoots of 2-week-old plantlets grown on MS–agar medium (Tables 2 and 3), in order to estimate genetically determined differences of carbohydrate status between accessions (Table 5). The results of this one-way ANOVA showed that all soluble sugar parameters measured at the end of day or at the end of night were significantly variable among the accessions, thus indicating significant genetic variation of carbohydrate status and metabolism (Table 5).
Table 5.
Variation in the biochemical parameters of the different accessions
| Trait | Genotype effect |
|---|---|
| GlcEoD | 7·5*** |
| SucEoD | 10·19*** |
| FruEoD | 3·32** |
| GlcEoN | 19·93*** |
| SucEoN | 2·23* |
| FruEoN | 15·12** |
Absolute sugar contents were measured as described in the Methods in shoots of plantlets grown for 2 weeks in the absence of atrazine. Soluble sugar contents were measured at the end of day (EoD) and at the end of night (EoN). The whole set of accessions and of data was analysed using a one-way ANOVA. *** P < 0·001; ** P < 0·01; * P < 0·05.
Correlation analysis of biochemical, physiological and stress response parameters
In order to identify potential relationships between biochemical and physiological traits characterizing atrazine tolerance or sensitivity, Pearson correlation analyses were performed on pairs of average values for physiological traits, which were measured in plantlets grown for 2 weeks in the absence of atrazine and transferred for 5 d in the absence or presence of 10 µm atrazine, and for quantifications of shoot endogenous soluble sugar levels, which were measured in 2-week-old plantlets prior to transfer.
The first Pearson coefficient matrix analysed correlations between physiological traits (Fv/Fm, chlorophyll and carotenoid contents) in the absence or presence of 10 µm atrazine (Table 6). No significant correlation at P < 0·05 was found between the level of development and functioning of the photosynthetic apparatus in the absence of atrazine and the response to atrazine stress. Correlations between physiological traits and biochemical parameters (endogenous sugars contents in shoots) in the absence of atrazine were also analysed (Table 7). No significant correlation at P < 0·05 was found between parameters of photosynthetic apparatus functioning (Fv/Fm, shoot chlorophyll content, shoot carotenoid content) in the absence of atrazine and parameters of soluble sugar levels in shoots at the end of day or at the end of night (Table 7). In contrast, a significant positive correlation was detected between shoot chlorophyll and carotenoid contents (R = 0·970) of plantlets in the absence of atrazine (Table 7). Moreover, Glc, Suc and Fru levels at the end of day in shoots were strongly and positively correlated with each other (Glc vs. Suc, R = 0·804; Glc vs. Fru, R = 0·803; and Suc vs. Fru, R = 0·841). The levels of reducing and total sugars were also highly correlated with all of the individual sugar levels. In contrast, at the end of night, no significant correlation was found between the different kinds of soluble sugars. Thus, whereas the level of total sugars at the end of day was correlated with levels of all three soluble sugars, the level of total sugars at the end of night was significantly correlated with Glc level only (Table 7). Finally, low correlations were detected between sugar levels at the end of day and sugar levels at the end of night. Glc levels at the end of day and at the end of night (R = 0·650), but not Fru or Suc levels, were significantly correlated. Levels of reducing and total sugars, whether at the end of day or at the end of night, also tended to be correlated, with R = 0·569 and R = 0·597, respectively
Table 6.
Correlation matrix (Pearson coefficients) of physiological traits in the absence or presence of 10 μm atrazine
| With atrazine |
|||
|---|---|---|---|
| Fv/Fm | Chlorophyll content | Carotenoid content | |
| Without atrazine | |||
| Fv/Fm | 0·391 | 0·167 | 0·176 |
| Chlorophyll content | 0·378 | 0·292 | 0·217 |
| Carotenoid content | 0·283 | 0·215 | 0·138 |
Absolute levels of physiological traits (Fv/Fm, shoot chlorophyll content and shoot carotenoid content) were measured as described in the Methods in plantlets grown for 2 weeks in the absence of atrazine and transferred for 5 d in the absence or presence of 10 µM atrazine. The whole set of accessions and of data was analysed. No significant correlation at P < 0·05 was found.
Table 7.
Correlation matrix (Pearson coefficients) of endogenous sugar levels and of physiological traits after transfer in the absence of atrazine
| Fv/Fm | Chlorophyll content | Carotenoid content | GlcEoD | SucEoD | FruEoD | Reducing sugarsEoD | Total sugarsEoD | GlcEoN | SucEoN | FruEoN | Reducing sugarsEoN | Total sugarsEoN | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fv/Fm | – | ||||||||||||
| Chlorophyll content | −0·298 | – | |||||||||||
| Carotenoid content | −0·327 | 0·970*** | – | ||||||||||
| GlcEoD | 0·223 | −0·202 | −0·185 | – | |||||||||
| SucEoD | 0·078 | 0·332 | 0·316 | 0·804** | – | ||||||||
| FruEoD | 0·101 | 0·028 | 0·024 | 0·803** | 0·841*** | – | |||||||
| Reducing sugarsEoD | 0·207 | −0·166 | −0·152 | 0·994*** | 0·835*** | 0·864*** | – | ||||||
| Total sugarsEoD | 0·171 | −0·002 | 0·002 | 0·967*** | 0·924*** | 0·890*** | 0·982*** | – | |||||
| GlcEoN | −0·072 | 0·248 | 0·297 | 0·650* | 0·635* | 0·401 | 0·623* | 0·651* | – | ||||
| SucEoN | 0·383 | −0·288 | −0·315 | −0·168 | -0·289 | −0·353 | −0·472 | −0·192 | −0·394 | – | |||
| FruEoN | −0·533 | 0·396 | 0·376 | 0·086 | 0·309 | 0·295 | 0·646* | 0·537* | 0·468 | −0·545 | – | ||
| Reducing sugarsEoN | −0·191 | 0·310 | 0·348 | 0·582 | 0·623* | 0·417 | 0·569 | 0·609* | 0·977*** | −0·472 | 0·646* | – | |
| Total sugarsEoN | −0·085 | 0·249 | 0·282 | 0·592 | 0·597 | 0·347 | 0·565 | 0·597 | 0·977*** | −0·192 | 0·537 | 0·956*** | – |
Absolute levels of physiological traits (Fv/Fm, shoot chlorophyll content and shoot carotenoid content) were measured as described in the Methods in plantlets grown for 2 weeks in the absence of atrazine and transferred for 5 d to control medium in the absence of atrazine. Absolute levels of endogenous sugars were measured as described in the Methods in shoots of plantlets grown for 2 weeks in the absence of atrazine. Soluble sugar contents were measured at the end of day (EoD) and at the end of night (EoN). The whole set of accessions and of data was analysed. *** P < 0·001; ** P < 0·01; * P < 0·05.
Correlations between biochemical parameters and physiological traits in the presence of 10 µm atrazine are presented in Table 8. Physiological parameters of atrazine-treated plantlets were found to show significant correlations between endogenous sugar parameters of plantlets prior to atrazine treatment. Indeed, the shoot carotenoid content was significantly and positively correlated with the level of Suc in shoots at the end of day (R = 0·639) and with the level of total sugars in shoots at the end of day (R = 0·596). Moreover, even if correlations between Suc level at the end of day and the other physiological traits were not significant, R-values were higher than those related to other sugar parameters at the end of day (Table 8). Physiological traits were not found to be correlated with the level of Suc at the end of night, but significant correlations were found with total sugar level at the end of night (total sugar EoN vs. chlorophyll content, R = 0·615; total sugar EoN vs. carotenoid content, R = 0·626). Moreover, there was a positive trend between Glc level at the end of night and the three physiological traits, with Pearson coefficients close to significance (Table 8). Finally, in the presence of 10 µm atrazine, there were high positive correlations between all the photosynthetic parameters. Indeed, Fv/Fm was correlated with chlorophyll and carotenoid contents with, respectively, Pearson coefficients of R = 0·884 and R = 0·862, while chlorophyll and carotenoid levels were correlated with a Pearson coefficient of R = 0·970.
Table 8.
Correlation matrix (Pearson coefficients) of endogenous sugar levels and of physiological traits after transfer in the presence of atrazine
| Fv/Fm | Chlorophyll content | Carotenoid content | |
|---|---|---|---|
| Fv/Fm | – | ||
| Chlorophyll content | 0·884*** | – | |
| Carotenoid content | 0·862*** | 0·970*** | – |
| GlcEoD | 0·342 | 0·343 | 0·527 |
| SucEoD | 0·467 | 0·502 | 0·639* |
| FruEoD | 0·247 | 0·353 | 0·518 |
| Reducing sugarsEoD | 0·335 | 0·355 | 0·542 |
| Total sugarsEoD | 0·393 | 0·419 | 0·596* |
| GlcEoN | 0·503 | 0·537 | 0·552 |
| SucEoN | 0·204 | 0·110 | 0·125 |
| FruEoN | 0·014 | 0·231 | 0·197 |
| Reducing sugarsEoN | 0·438 | 0·520 | 0·524 |
| Total sugarsEoN | 0·556 | 0·615* | 0·626* |
Absolute levels of physiological traits (Fv/Fm, shoot chlorophyll content and shoot carotenoid content) were measured as described in the Methods in plantlets grown for 2 weeks in the absence of atrazine and transferred for 5 d to control medium in the presence of 10 µm atrazine. Absolute levels of endogenous sugars were measured as described in the Methods in shoots of plantlets grown for 2 weeks in the absence of atrazine. Soluble sugar contents were measured at the end of day (EoD) and at the end of night (EoN). The whole set of accessions and of data was analysed. * Significant correlation at P < 0·05, *** P < 0·001.
Principal component analysis
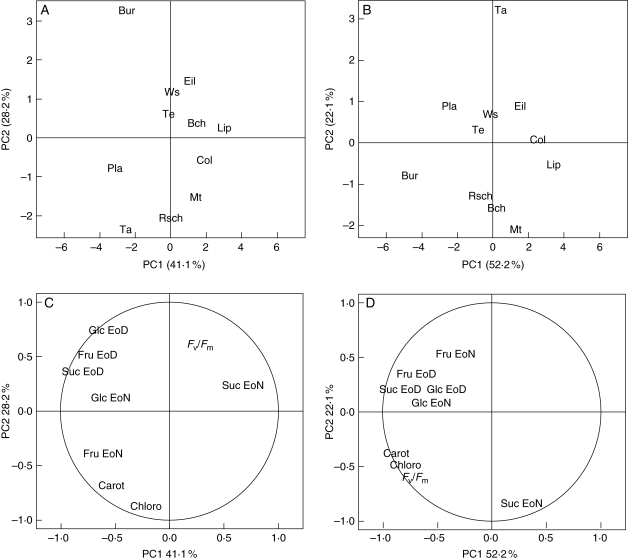
In order to classify accessions according to their response to atrazine and to their carbohydrate status, a PCA was carried out on the whole set of average values for endogenous soluble sugar levels in shoots prior to transfer and for physiological traits after transfer in the absence or presence of 10 µm atrazine.
Figure 4 shows that the first factorial plane, defined by PC1 and PC2 axes, respectively, accounted for 41·1 and 28·2 % of the observed variation, in the absence of atrazine (Fig. 4A), and for 52·2 and 22·1 % of the observed variation, in the presence of 10 µm atrazine (Fig. 4B).
Fig. 4.
Principal component analysis of endogenous soluble sugars and xenobiotic stress responses of Arabidopsis thaliana accessions. Principal component analysis was performed on the correlation matrix of averages of biochemical parameters [absolute levels of endogenous soluble sugars in shoots at the end of day (EoD) and at the end of night (EoN)] and of physiological traits (absolute levels of Fv/Fm, shoot chlorophylls and shoot carotenoids) measured on the 11 accessions. Soluble sugars were measured in shoots of 2-week-old plantlets prior to atrazine treatment (Tables 2 and 3) and physiological traits were measured after 5 d of transfer onto fresh medium in the absence or presence of 10 µm atrazine. (A) and (B) represent the repartition of accessions in the absence or presence of 10 µM atrazine, respectively. (C) and (D) represent the distribution of variables on the first plane (PC1 and PC2) in the absence or presence of 10 µm atrazine, respectively.
As shown in Fig. 4C by the correlation circle, in the absence of atrazine, the first axis was essentially explained by shoot Suc level at the end of day, shoot Fru level at the end of day and shoot Glc level at the end of night, and the second axis was essentially explained by the three physiological traits and by shoot Glc level at the end of day. The localization of Ta-0, Rsch-0, Mt-0 and Pla-0 (Fig. 4A) indicated high levels of chlorophyll and carotenoid in shoots of these three accessions in the absence of atrazine, in contrast to Bur-0, which showed the opposite situation, and was characterized by a high level of Glc at the end of day. The other accessions had a central position characterized by intermediate values.
As shown in Fig. 4D by the correlation circle, in the presence of atrazine, the first axis was essentially explained by the physiological traits and sugar contents, except for the Fru and Suc level in shoots at the end of night. However, the variable that most contributed to this axis was the shoot Suc level at the end of day. The second axis was mainly explained by the shoot Suc level at the end of night. Bur-0, Pla-0 and Ta-0 were found to be at outlying positions (Fig. 4B). Bur-0 and Pla-0 were characterized by a high level of Suc at the end of day in shoots and low sensitivity to atrazine, while Ta-0 presented a very low level of Suc in shoots at the end of night and a high level of Fru in shoots at the end of night. The group of Rsch-0, Bch-1 and Mt-0 accessions was characterized by high levels of Suc at the end of night. Another group, including Eil-0, Col-0 and Lip-0, was characterized by low sugar levels and high sensitivity to atrazine (Fig. 4B). The central position of Te-0 and Ws accessions revealed intermediate values for all of the measured parameters.
Natural variation of atrazine-induced ROS accumulation in arabidopsis plantlets
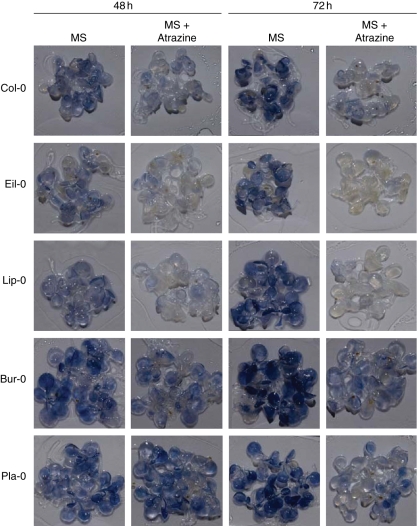
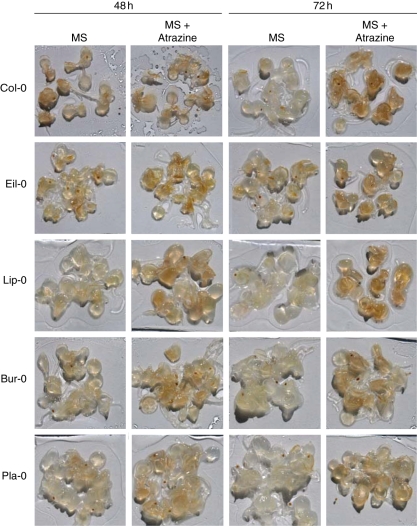
A 5-d treatment in the presence of 10 µm atrazine reveals major injuries of the photosynthetic apparatus and of the root system under conditions of atrazine sensitivity. However, earlier modifications, such as ROS accumulation, may determine the eventual sensitivity or tolerance to atrazine. In order to determine whether endogenous variations of soluble sugars were related to differences in the accumulation of ROS, 2-week-old A. thaliana plantlets were transferred to MS medium in the absence or presence of 10 µm atrazine for 48 and 72 h. In vivo detection of hydrogen peroxide and superoxide anion was carried out on plantlets of the different accessions, which showed a continuum of responses (data not shown). Typical accessions, Bur-0, Pla-0, Eil-0, Col-0 and Lip-0, which showed constrasted ROS accumulation in response to atrazine, are shown in Figs 5 and 6.
Fig. 5.
Analysis of superoxide anion accumulation by NBT staining in plantlets of different accessions grown for 2 weeks in the absence of atrazine and transferred for 48 or 72 h in the absence or presence of 10 µm atrazine.
Fig. 6.
Analysis of hydrogen peroxide accumulation by DAB staining in plantlets of different accessions grown for 2 weeks in the absence of atrazine and transferred for 48 or 72 h in the absence or presence of 10 µm atrazine.
Superoxide anion (O2·−) detection was performed using the NBT staining method as described in Materials and methods. In the absence of atrazine, the levels of superoxide anion were variable between the five accessions under study (Fig. 5). Although accumulation of superoxide was detectable at 48 and 72 h for all of the accessions, the basal levels of superoxide in plantlets grown on MS medium were low in Eil-0 and Lip-0 accessions, intermediate in Col-0, and high in Bur-0 and Pla-0 accessions. The presence of 10 µm atrazine led to a strong decrease of superoxide content, which, in atrazine-sensitive plantlets (Eil-0, Col-0 and Lip-0), was more accentuated at 72 h than at 48 h. In contrast, plantlets of accessions (Bur-0 and Pla-0) showing lower sensitivity to atrazine succeeded in maintaining a significant level of superoxide content in the presence of atrazine.
H2O2 detection was performed using the DAB staining method as described in Materials and methods. All of the five accessions showed H2O2 accumulation in the absence or presence of atrazine (Fig. 6). After 48 h of transfer in the absence of atrazine, H2O2 contents were low, and little variation was observed between the five accessions, except for Col-0 and Eil-0 plantlets, which showed slightly higher H2O2 basal content. A slight discoloration was observed between 48 and 72 h of control treatment for all accessions, except for Eil-0. The presence of atrazine was found to induce accumulation of H2O2 at 48 and 72 h of treatment, with higher H2O2 contents in plantlets of atrazine-sensitive accessions (Eil-0, Lip-0 and Col-0) than in plantlets of accessions (Bur-0 and Pla-0) showing lower atrazine sensitivity.
DISCUSSION
Natural variation of absolute levels of shoot endogenous soluble sugars under non-stress conditions
Recent studies have identified and characterized natural variation of carbohydrate contents and metabolic activities in Arabidopsis under non-stress conditions (Calenge et al., 2006; Cross et al., 2006; Davey et al. 2008). Thus, carbohydrate contents in rosette leaves were highly variable among recombinant inbred lines (Calenge et al., 2006) or among natural accessions (Cross et al., 2006). These differences of carbohydrate contents may be related to variations of photosynthetic activity, variations of carbon allocation between storage, growth or respiration, or variations of carbon utilization. The Bur-0 accession has thus been characterized, among a set of 24 accessions, by high sugar content, high starch content and low amino acid content in rosette leaves, thus indicating an imbalance between C and N metabolism (Cross et al., 2006). However, Cross et al. (2006) pointed out that, depending on light and mineral nutrition conditions, growth and development could be correlated with absolute carbohydrate levels (Calenge et al., 2006), or with enzyme activities and metabolic fluxes in central C and N metabolism (Cross et al., 2006). The set of natural accessions in the present work was chosen from these previous publications on potential relationships between variations of endogenous sugar contents and growth and development.
Although growth conditions involved a 16 h daylength, the low level of light was likely to result in carbon limitation. These conditions (low light, long day) were identical to those of our previous studies (Ramel et al., 2007, 2009), where carbon limitation was used to highlight the effects of exogenously added sucrose. Under these conditions, the different accessions showed contrasted patterns of night–day fluctuations of soluble sugar levels (Tables 2 and 3), with some accessions, such as Col-0, showing no variation between end of day and end of night soluble sugar levels. Previous studies (Matt et al., 1998; Gibon et al., 2004) had indicated that fluctuations of soluble sugar levels at the end of day and at the end of night depend on light growth conditions. Parallel analysis of low light–long day and higher light–long day conditions was therefore carried out, and revealed that Col-0 plantlets under higher light conditions showed normal fluctuations of low and high levels of soluble sugars, respectively, at the end of night and at the end of day (data not shown). Further work should determine whether this unexpected lack of night–day fluctuations of soluble sugar levels under low light, carbon-limiting conditions is compounded at early stages of development of the photosynthetic apparatus, and of carbon allocation and utilization pathways.
At any rate, growth under low light–long day conditions was useful to enhance differences of endogenous sugar levels between accessions, and for the comparison of results with the effects of exogenous sugars (Ramel et al., 2007, 2009). Moreover, the study of plantlets under conditions of low light is likely to be relevant to environmental situations where xenobiotic application may affect seedling establishment during springtime.
Absolute carbohydrate levels in shoots, whether at the end of day or at the end of night, were not found to be correlated with PSII efficiency (Fv/Fm), chlorophyll levels or carotenoid levels in plantlets under non-stress conditions (Table 7). This may agree with the absence of correlation between soluble carbohydrate levels and growth (Cross et al., 2006). On the other hand, the analysis of superoxide content in plantlets of the different accessions grown on control medium showed higher levels of superoxide in the Bur-0 accession (Fig. 5). Superoxide radical is formed mainly by reduction of oxygen at the PSI site (Asada et al., 1974), with oxygen serving as an electron acceptor. Higher levels of superoxide may thus reflect higher activity in PSI, and could be interpreted as a result of higher cyclic photophosphorylation activity in the Bur-0 accession.
Natural variation of pre-stress endogenous soluble sugars is related to major traits of xenobiotic and oxidative stress tolerance
The protective roles of soluble sugars under different kinds of stress conditions (Sulmon et al., 2004; Loreti et al., 2005; Gomez-Ariza et al., 2007; Ramel et al., 2007; Banti et al., 2008; Morkunas and Bednarksi, 2008) have been demonstrated by exogenous sugar pre-treatment or by continuous supply of exogenous sugars. However, endogenous Suc levels in Arabidopsis leaves vary between 1 and 3 mm (Gonzali et al., 2006), and are thus lower than Suc levels resulting from exogenous sugar treatments (Graham et al., 1994; Martin et al., 2002; Sulmon et al., 2004; Loreti et al., 2005; Ramel et al., 2007). It thus remained unclear whether endogenous levels of soluble sugars played an important role in stress responses.
The different modalities of atrazine treatment revealed significant differences of sensitivity to various atrazine concentrations between the different accessions (Figs 1–3). Several accessions, such as the Bur-0 accession, were much less sensitive to atrazine stress. Moreover, the present set of natural accessions, chosen on the basis of their varying sugar contents, pointed to a qualitative link between pre-stress endogenous sugar levels in shoots and the response of plantlets to subsequent xenobiotic stress. Accessions showing the highest basic levels of endogenous sugars were generally less sensitive to atrazine stress. Statistical analysis (Table 8) revealed consistent trends and significant correlations between improved tolerance to atrazine treatment and various traits of carbohydrate metabolism, especially the level of total sugars at the end of the night, the level of total sugars at the end of the day and the level of sucrose at the end of the day.
A recent study (Korn et al., 2008) has demonstrated a strong relationship between freezing tolerance and the overaccumulation of different sugars as compatible solutes in A. thaliana accessions. The present work outlines a novel kind of relationship, between pre-stress carbohydrate metabolism and subsequent xenobiotic and oxidative stress, that does not involve overaccumulation of compatible solutes.
This relationship may have been enhanced by the carbon-limiting conditions of low light that were applied to plantlets, and may be less pronounced under high light conditions. Nevertheless, situations of multiple stresses, such as combination of low light and abiotic stressors, are relevant to environmental conditions (Mittler, 2006). Thus, the demonstration of this relationship between endogenous carbohydrate metabolism and stress response points to an important integration of nutritional status and stress tolerance in plantlets.
Involvement of pre-stress endogenous soluble sugars in xenobiotic stress tolerance
Parallel transcriptomic and biochemical analysis of control, atrazine alone, Suc alone and atrazine plus Suc treatments had shown important interactions of atrazine and Suc effects, with early induction of specific transcription factors (Ramel et al., 2007, 2009). In these previous studies, the effects of immediate atrazine exposure interacted with endogenous sugar levels that were determined by the exogenous treatment. It could therefore be reasoned that, in the present work, where pre-stress endogenous sugar levels were correlated with subsequent atrazine tolerance, atrazine first interacted with pre-existing endogenous sugar levels, and that the effects of this primary interaction were important for induction and further development of tolerance mechanisms.
Atrazine-related stress is light and photosynthesis dependent, and involves imbalance of ROS dynamics, with accumulation of singlet oxygen (Rutherford and Krieger-Liszkay, 2001; Ramel et al., 2009), a decrease of superoxide levels (Ramel et al., 2009) and accumulation of hydrogen peroxide (Ramel et al., 2009). The differences between accessions in terms of pre-stress shoot endogenous sugar levels and of stress responses were shown to be linked to important effects on hydrogen peroxide and superoxide dynamics (Figs 5 and 6). Thus, H2O2 accumulation in the presence of atrazine (Fig. 6) was decreased in accessions that were the least sensitive to atrazine. Analysis of superoxide radical levels (Fig. 5) also showed that superoxide levels were maintained in the accessions that were the least sensitive to atrazine. Atrazine binding to D1 protein of PSII and inhibition of electron feeding to PSI block oxygen partial reduction at the PSI level and decrease superoxide radical production. The maintenance of superoxide radical levels in the Bur-0 and Pla-0 accessions could thus be ascribed to maintenance of photosynthetic activity in the presence of atrazine. All of these effects on ROS dynamics in accessions with various pre-stress endogenous sugar levels in shoots were similar to the positive effects of exogenous Suc on ROS dynamics (Ramel et al., 2009), and were in agreement with the importance of pre-stress carbon nutritional status, as discussed above.
Defence systems are generally thought to be costly in terms of carbon and energy investment (Rhoades, 1979). Stress responses and response-associated costs are thus likely to increase only when the benefits outweigh the cost of tolerance response (Zangerl and Bazzaz, 1992; Karban and Baldwin, 1997). It is reasonable to assume that efficient investment in stress responses requires decision-like mechanisms, whereby the plant can sense available carbon resources and reorchestrate metabolic and stress defence processes in response to environnemental cues. The positive effects of pre-stress endogenous sugar levels on tolerance to atrazine may be related to such mechanisms.
Pre-stress Suc level was the only single soluble sugar parameter that was found to be positively correlated with greater atrazine tolerance (Table 8). Suc, as a central metabolite between photosynthesis, carbon transport and carbon utilization (Salerno and Curatti, 2003), plays an important role in the regulation of carbon allocation (Chiou and Bush, 1998). Exogenous treatment studies have also shown that, in the tolerance to anoxic stress (Loreti et al., 2005) and to atrazine stress (Sulmon et al., 2004, 2006), Suc treatment was much more efficient than Glc treatment. Finally, exogenous Suc has been shown to induce in a specific way a number of stress-related genes (Solfanelli et al., 2006). These Suc-specific processes may be due to Suc-specific signalling pathways, which remain to be characterized (Couée et al., 2006), or to better uptake or utilization of exogenous Suc, when compared with Glc (Banti et al., 2008).
Analysis of natural endogenous variations avoids such differential effects related to uptake and metabolic utilization of exogenous molecules. The specificity of Suc among the various endogenous biochemical traits of carbohydrate metabolism therefore established the importance of Suc as an endogenous player of stress defence regulations and tolerance. It further showed that the correlation with improved atrazine tolerance could not be primarily ascribed to intrinsic reducing properties of soluble sugars, since Suc is a non-reducing disaccharide. The correlation between pre-stress Suc level at the end of the day and greater atrazine tolerance may therefore reflect adaptive mechanisms that link Suc accumulation, photosynthesis-related stress and induction of stress defences. Characterization of the natural variation of these mechanisms will imply further work involving quantitative trait locus analysis.
Differential impact of xenobiotics on natural plant populations
Because of its widespread use and of its persistence, atrazine has been detected as a common contaminant in soils, streams, rivers and lakes (Solomon et al., 1996; Clark et al., 1999). Numerous studies have shown the effects of this herbicide on reduction of photosynthesis, chlorophyll synthesis, cell growth and nitrogen fixation in aquatic photosynthetic communities (Millie and Hersh, 1987; Hersh and Crumpton, 1989; Weiner et al., 2007).
Atrazine pollution can range from 0·5 nm to 0·14 µm in surface waters of river and urban basins, and peaks ranging from 0·3 to 1·4 µm can be reached in agricultural basins (Weiner et al., 2007). In the present study, sub-lethal or lethal atrazine concentrations were in the range (from 0·25–1 µm) of runoff pollutions in agricultural basins, or at the level of acute lethal exposure (10 µm).
Natural variation of sensitivity in Arabidopsis accessions (Figs 1–3) was shown to occur under all of these concentrations. The lowest atrazine concentration of 0·25 µm negatively affected primary root growth of plantlets (Fig. 2). The different accessions also showed important differences of sensitivity during exposure to a high, lethal, atrazine concentration (Fig. 3). It thus seemed that even a low level of runoff atrazine pollution could have an impact on seedling establishment of natural populations, and exert selective effects on specific genotypes. Since such levels of pollution, or even higher levels of pollution, may occur in basins of intensive agriculture, further work is required in order to determine whether herbicide runoff pollution could have large-scale effects on the population dynamics of wild plant species, and what consequences such effects could have on the functioning of terrestrial ecosystems.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part by the interdisciplinary programme ‘Ingénierie écologique’ (Centre National de la Recherche Scientifique, France) and by a fellowship (to F.R.) from the Ministère de l'Enseignement Supérieur et de la Recherche (France). We also wish to thank Irène Hummel for critical reading and fruitful discussions.
LITERATURE CITED
- Asada K, Kiso K, Yoshikawa K. Univalent reduction of molecular oxygen by spinach chloroplasts on illumination. Journal of Biological Chemistry. 1974;249:2175–2181. [PubMed] [Google Scholar]
- Banti V, Loreti E, Novi G, Santaniello A, Alpi A, Perata P. Heat acclimation and cross-tolerance against anoxia in Arabidopsis. Plant, Cell and Environment. 2008;31:1029–1037. doi: 10.1111/j.1365-3040.2008.01816.x. [DOI] [PubMed] [Google Scholar]
- Boyes DC, Zayed AM, Ascenzi R, et al. Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. The Plant Cell. 2001;13:1499–1510. doi: 10.1105/TPC.010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calenge F, Saliba-Colombani V, Mahieu S, Loudet O, Daniel-Vedele F, Krapp A. Natural variation for carbohydrate content in Arabidopsis. Interaction with complex traits dissected by quantitative genetics. Plant Physiology. 2006;141:1630–1643. doi: 10.1104/pp.106.082396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou TJ, Bush DR. Sucrose is a signal molecule in assimilate partitioning. Proceedings of the National Academy of Sciences, USA. 1998;95:4784–4788. doi: 10.1073/pnas.95.8.4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark GM, Goolsby DA, Battaglin WA. Seasonal and annual load of herbicides from the Mississippi River basin to the Gulf of Mexico. Environmental Science and Technology. 1999;33:981–986. [Google Scholar]
- Couée I, Sulmon C, Gouesbet G, El Amrani A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. Journal of Experimental Botany. 2006;57:449–459. doi: 10.1093/jxb/erj027. [DOI] [PubMed] [Google Scholar]
- Cross JM, von Korff M, Altmann T, et al. Variation of enzyme activities and metabolite levels in 24 arabidopsis accessions growing in carbon-limited conditions. Plant Physiology. 2006;142:1574–1588. doi: 10.1104/pp.106.086629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey MP, Burrell MM, Woodward FI, Quick WP. Population-specific metabolic phenotypes of Arabidopsis lyrata ssp. petraea. New Phytologist. 2008;177:380–388. doi: 10.1111/j.1469-8137.2007.02282.x. [DOI] [PubMed] [Google Scholar]
- Fryer MJ, Oxborough K, Mullineaux PM, Baker NR. Imaging of photo-oxidative stress responses in leaves. Journal of Experimental Botany. 2002;53:1249–1254. [PubMed] [Google Scholar]
- Gibon Y, Blasing OE, Palacios-Rojas N, et al. Adjustment of diurnal starch turnover to short days: depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars and post-translational activation of ADP-glucose pyrophosphorylase in the following light period. The Plant Journal. 2004;39:847–862. doi: 10.1111/j.1365-313X.2004.02173.x. [DOI] [PubMed] [Google Scholar]
- Gomez-Ariza J, Campo S, Rufat M, et al. Sucrose-mediated priming of plant defense responses and broad-spectrum disease resistance by overexpression of the maize pathogenesis-related PRms protein in rice plants. Molecular Plant-Microbe Interactions. 2007;20:832–842. doi: 10.1094/MPMI-20-7-0832. [DOI] [PubMed] [Google Scholar]
- Gonzali S, Loreti E, Solfanelli C, Novi G, Alpi A, Perata P. Identification of sugar-modulated genes and evidence for in vivo sugar sensing in Arabidopsis. Journal of Plant Research. 2006;119:115–123. doi: 10.1007/s10265-005-0251-1. [DOI] [PubMed] [Google Scholar]
- Graham IA, Denby KJ, Leaver CJ. Carbon catabolite repression regulates glyoxylate cycle gene-expression in cucumber. The Plant Cell. 1994;6:761–772. doi: 10.1105/tpc.6.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh CM, Crumpton WG. Atrazine tolerance of algae isolated from 2 agricultural streams. Environmental Toxicology and Chemistry. 1989;8:327–332. [Google Scholar]
- Karban R, Baldwin IT. Induced responses to herbivory. University of Chicago Press; 1997. Chicago. [Google Scholar]
- Koornneef M, Alonso-Blanco C, Vreugdenhil D. Naturally occurring genetic variation in Arabidopsis thaliana. Annual Review of Plant Biology. 2004;55:141–172. doi: 10.1146/annurev.arplant.55.031903.141605. [DOI] [PubMed] [Google Scholar]
- Korn M, Peterek S, Mock HP, Heyer AG, Hincha DK. Heterosis in the freezing tolerance, and sugar and flavonoid contents of crosses between Arabidopsis thaliana accessions of widely varying freezing tolerance. Plant, Cell and Environment. 2008;31:813–827. doi: 10.1111/j.1365-3040.2008.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK, Wellburn AR. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochemical Society Transactions. 1983;11:591–592. [Google Scholar]
- Loreti E, Poggi A, Novi G, Alpi A, Perata P. A genome-wide analysis of the effects of sucrose on gene expression in Arabidopsis seedlings under anoxia. Plant Physiology. 2005;137:1130–1138. doi: 10.1104/pp.104.057299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T, Oswald O, Graham IA. Arabidopsis seedling growth, storage lipid mobilization, and photosynthetic gene expression are regulated by carbon:nitrogen availability. Plant Physiology. 2002;128:472–481. doi: 10.1104/pp.010475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt P, Schurr U, Klein D, Krapp A, Stitt M. Growth of tobacco in short-day conditions leads to high starch, low sugars, altered diurnal changes in the Nia transcript and low nitrate reductase activity, and inhibition of amino acid synthesis. Planta. 1998;207:27–41. doi: 10.1007/s004250050452. [DOI] [PubMed] [Google Scholar]
- Millie DF, Hersh CM. Statistical characterizations of the atrazine-induced photosynthetic inhibition of Cyclotella meneghiniana (Bacillariophyta) Aquatic Toxicology. 1987;10:239–249. [Google Scholar]
- Mittler R. Abiotic stress, the field environment and stress combination. Trends in Plant Science. 2006;11:15–19. doi: 10.1016/j.tplants.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Morkunas I, Bednarski W. Fusarium oxysporum-induced oxidative stress and antioxidative defenses of yellow lupine embryo axes with different sugar levels. Journal of Plant Physiology. 2008;165:262–277. doi: 10.1016/j.jplph.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Price J, Laxmi A, St Martin SK, Jang JC. Global transcription profiling reveals multiple sugar signal transduction mechanisms in Arabidopsis. The Plant Cell. 2004;16:2128–2150. doi: 10.1105/tpc.104.022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel F, Sulmon C, Cabello-Hurtado F, et al. Genome-wide interacting effects of sucrose and herbicide-mediated stress in Arabidopsis thaliana: novel insights into atrazine toxicity and sucrose-induced tolerance. BMC Genomics. 2007;8:450. doi: 10.1186/1471-2164-8-450. doi:10.1186/1471-2164-8-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel F, Sulmon C, Bogard M, Couée I, Gouesbet G. Differential dynamics of reactive oxygen species and antioxidative mechanisms during atrazine injury and sucrose-induced tolerance in Arabidopsis thaliana plantlets. BMC Plant Biology. 2009;9:28. doi: 10.1186/1471-2229-9-28. doi:10.1186/1471-2229-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MV, Davis KR. Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: the role of salicylic acid. The Plant Journal. 1999;17:603–614. doi: 10.1046/j.1365-313x.1999.00400.x. [DOI] [PubMed] [Google Scholar]
- Rhoades DF. Evolution of plant chemical defense against herbivores. In: Rosenthal GA, Jantzen DH., editors. Herbivores: their interaction with secondary plant metabolites. New York: Academic Press; 1979. pp. 1–55. [Google Scholar]
- Rutherford AW, Krieger-Liszkay A. Herbicide-induced oxidative stress in photosystem II. Trends in Biochemical Sciences. 2001;26:648–653. doi: 10.1016/s0968-0004(01)01953-3. [DOI] [PubMed] [Google Scholar]
- Salerno GL, Curatti L. Origin of sucrose metabolism in higher plants: when, how and why? Trends in Plant Science. 2003;8:63–69. doi: 10.1016/S1360-1385(02)00029-8. [DOI] [PubMed] [Google Scholar]
- Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P. Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiology. 2006;140:637–646. doi: 10.1104/pp.105.072579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon KR, Baker DB, Richards RP, et al. Ecological risk assessment of atrazine in North American surface waters. Environmental Toxicology and Chemistry. 1996;15:31–74. doi: 10.1002/etc.2050. [DOI] [PubMed] [Google Scholar]
- Strand A, Hurry V, Henkes S, et al. Acclimation of Arabidopsis leaves developing at low temperatures. Increasing cytoplasmic volume accompanies increased activities of enzymes in the Calvin cycle and in the sucrose-biosynthesis pathway. Plant Physiology. 1999;119:1387–1397. doi: 10.1104/pp.119.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulmon C, Gouesbet G, Couee I, El Amrani A. Sugar-induced tolerance to atrazine in Arabidopsis seedlings: interacting effects of atrazine and soluble sugars on psbA mRNA and D1 protein levels. Plant Science. 2004;167:913–923. [Google Scholar]
- Sulmon C, Gouesbet G, El Amrani A, Couee I. Sugar-induced tolerance to the herbicide atrazine in Arabidopsis seedlings involves activation of oxidative and xenobiotic stress responses. Plant Cell Reports. 2006;25:489–498. doi: 10.1007/s00299-005-0062-9. [DOI] [PubMed] [Google Scholar]
- Sulmon C, Gouesbet G, Binet F, Martin-Laurent F, El Amrani A, Couee I. Sucrose amendment enhances phytoaccumulation of the herbicide atrazine in Arabidopsis thaliana. Environmental Pollution. 2007;145:507–515. doi: 10.1016/j.envpol.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Thum KE, Shin MJ, Palenchar PM, Kouranov A, Coruzzi GM. Genome-wide investigation of light and carbon signaling interactions in Arabidopsis. Genome Biology. 2004;5(2):R10. doi: 10.1186/gb-2004-5-2-r10. doi: 10.1186/gb-2004-5-2-r10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JA, DeLorenzo ME, Fulton MH. Atrazine induced species-specific alterations in the subcellular content of microalgal cells. Pesticide Biochemistry and Physiology. 2007;87:47–53. [Google Scholar]
- Zangerl A, Bazzaz F. Theory and pattern in plant defense allocation. In: Fritz RS, Simms EL, editors. Plant resistance to herbivores and pathogens, ecology, evolution and genetics. Chicago, IL: University of Chicago Press; 1992. pp. 363–391. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.