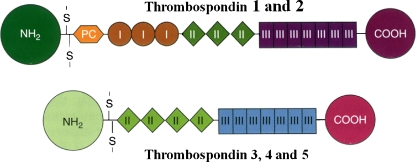
Fig. 1.
A schematic representation of the structures of the individual chains in the thrombospondins. TSP-1 and TSP-2 are trimers and TSP-3, -4, and -5 are pentamers. The sizes and amino acid sequences of the NH2-terminal domains vary considerably among the TSPs; in the case of TSP-5 this domain consists of only a few amino acids. The oligomerization domain, containing the interchain disulfide bonds, is followed by a procollagen homology domain (PC), also known as a von Willebrand type C repeat, and in the case of TSP-1 and TSP-2, by three type I (thrombospondin structural or properdin-like) repeats. All TSPs have type II (EGF-like) and type III (calcium binding) repeats, and a COOH-terminal domain. Figure and legend are reproduced with permission from Bornstein, P. ‘Matricellular Proteins’ in Encyclopedia of Respiratory Medicine , G.J Laurent and S.D. Shapiro, Eds. Elsevier Limited, Oxford, UK; Volume 2, pp 175–183, 2006