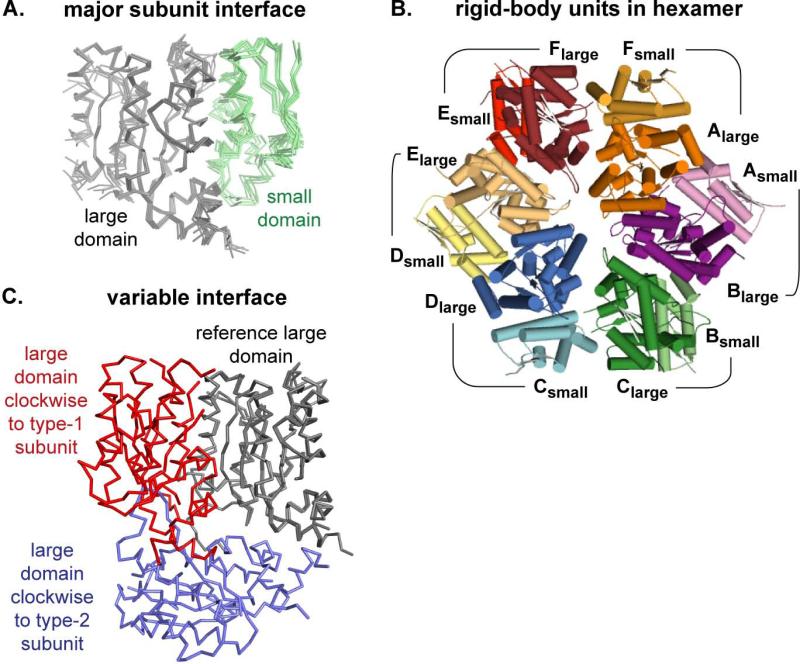
Figure 2. Subunit-Subunit Interfaces.
(A) The large AAA+ domains (grey; Cα trace) from all six subunits of the nucleotide-bound hexamer were superimposed, showing highly conserved positions of the small AAA+ domain (green) in the counter-clockwise subunit. The same orientation of adjacent large and small AAA+ domains was seen in nucleotide-free ClpX, suggesting that these neighboring domains comprise a rigid-body unit.
(B) The six rigid-body units in the nucleotide-bound ClpX hexamer (top view; cartoon representation) are shown in different colors, with the small AAA+ domain of each rigid-body unit colored a lighter shade (e.g., Asmall; light purple) than the clockwise large AAA+ domain in the same unit (e.g., Blarge; dark purple).
(C) Modules consisting of two adjacent large AAA+ domains from the nucleotide-bound hexamer were compared by superimposing the B domain of the B/C module on the C domain of the C/D module. This alignment shows that a large AAA+ domain clockwise from a type-1 subunit (red) occupies a very different position from a large AAA+ domain clockwise to a type-2 subunit (blue).