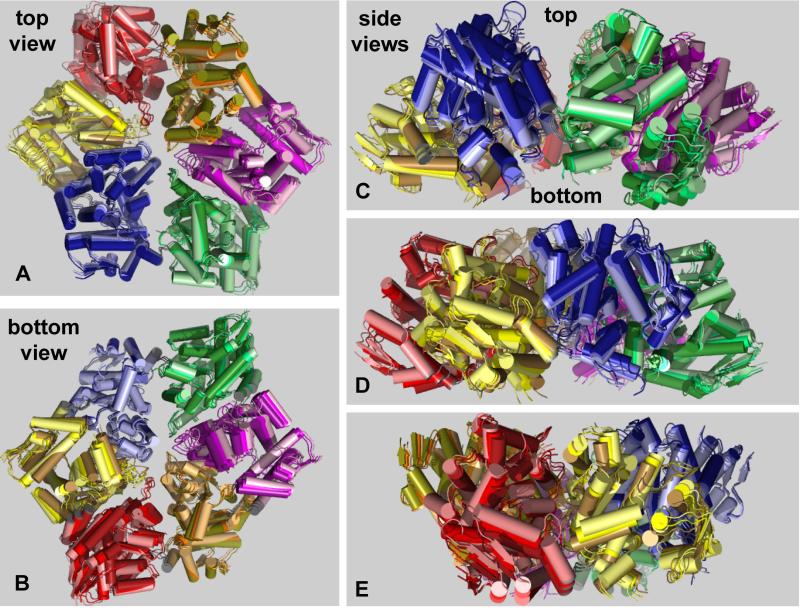
Figure 4. Nucleotide-Dependent Motions.
(A) Top, (B) bottom, and side views (C-E) of the nucleotide-free and nucleotide-bound ClpX hexamers in cartoon representation following superposition. An intermediate structure obtained by averaging is also shown. For each structure, each of the six rigid-body units is a different color. Within each unit, lighter shades represent the nucleotide-free structure, intermediate shades represent the averaged structure, and darker shades represent the nucleotide-bound structure. Thus, motions induced by nucleotide binding progress from lighter to darker shades. Asymmetry of the nucleotide-free and nucleotide-bound hexamers is evident in the top and bottom views (looking along the axis of the pore) and in the side views. The views in panel B and C were generated from that in panel A by 180° and 90° rotations, respectively, around the x-axis. The views shown in panels D and E represent rotations around the y-axis in 50° and 75° increments from the view in panel C.