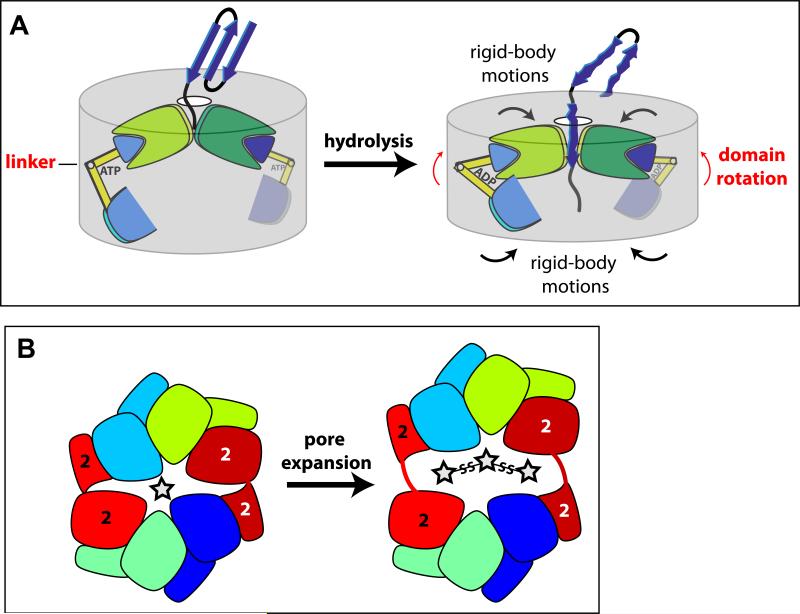
Figure 6. Models for protein unfolding and pore expansion.
(A) Cartoon showing how ATP hydrolysis might change rotations between the large and small AAA+ domains of two ClpX subunits. These domain-domain rotations, in turn, could drive rigid-body movements that result in unfolding and translocation of a bound native substrate.
(B) The cartoon on the left shows that the ClpX hexamer can be viewed as consisting of two jaw-like elements. The main contacts between these jaws are formed by the interfaces between the large and small AAA+ domains of the type-2 subunits (red/dark red). Opening of these interfaces, as shown in the exaggerated right cartoon, provides a potential mechanism for pore expansion to accommodate large substrates, including those with multiple chains.