Fig. 1.
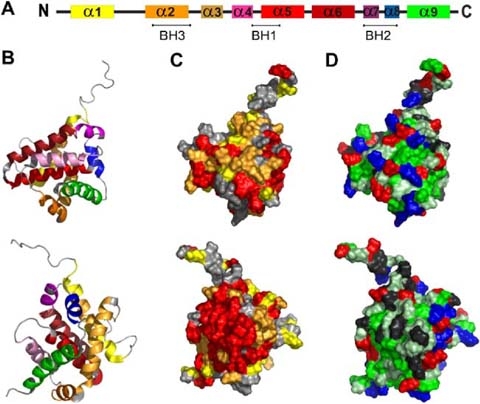
Representation of conserved regions, and hydrophobicity in the Bax structure. The secondary as well as a cartoon of the ternary structure of Bax (PDB:1F16) are shown in a and b, respectively. Helices are colored according to the code: α1 yellow, α2 fawn, α3 orange, α4 pink, α5 light red, α6 dark red, α7 purple, α8 blue, α9 green. c Shows a representation of the sequence conservation of the Bax surface. Conserved amino acids were identified by ClustalW alignment of human Bax alpha and its orthologs of Xenopus laevis and Danio rerio and were highlighted in the published NMR structure of human Bax alpha (Suzuki et al. 2000). Amino acids identical in all sequences are shown in red. Substitutions which were classified as highly conserved residues are marked in orange and semi-conserved ones are displayed in yellow. d Demonstrates the hydrophobicity of the surface exposed amino acids (according to the hydropathy index by Kyte and Doolittle 1982). Acidic [E,D] and basic [K,R] amino acids are shown in red and blue, respectively. Hydrophobic amino acids [I,V,L,A,C,F,M] are shown in green and slightly hydrophobic residues [G,T,S,W] in light green. Others [Q,N,P,H,Y] are marked in grey. b–d Show two views of the molecule tilted by 180°
