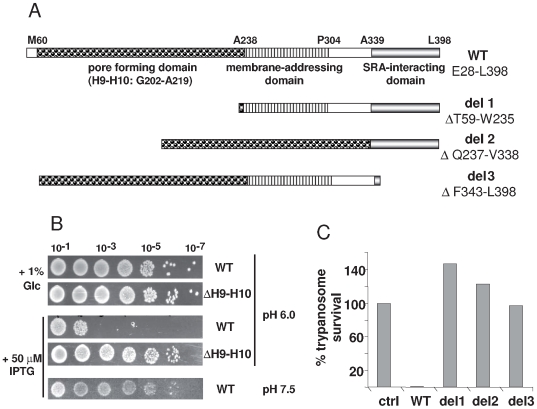
Figure 1. Lytic activities of apoL1.
A. Schematic representation of apoL1 and apoL1 variants analyzed in this work, with indication of the amino acid residues delineating the three protein domains (see ref. 4 for details). B. Colicin-like activity of WT and mutant apoL1. pCDF-DUET plasmids encoding apoL1 variants provided with a C-terminal V5-His6 tag were transfected into E. coli BL21(DE3). In the case of the deletion (del) variants, an N-terminal bacterial signal peptide (pelB) was added. After overnight incubation at 37°C the bacterial plating efficiency was scored comparing expression induction by IPTG addition or non-induction by glucose (Glc) addition, or comparing the effect of pH. The ΔH9–H10 apoL1 mutant lacks the region encoding helices 9 and 10 of the pore-forming domain. C. Trypanolytic potential of various apoL1 variants, as determined on T. b. brucei after 24 h–incubation in vitro (ctrl = control without apoL1).