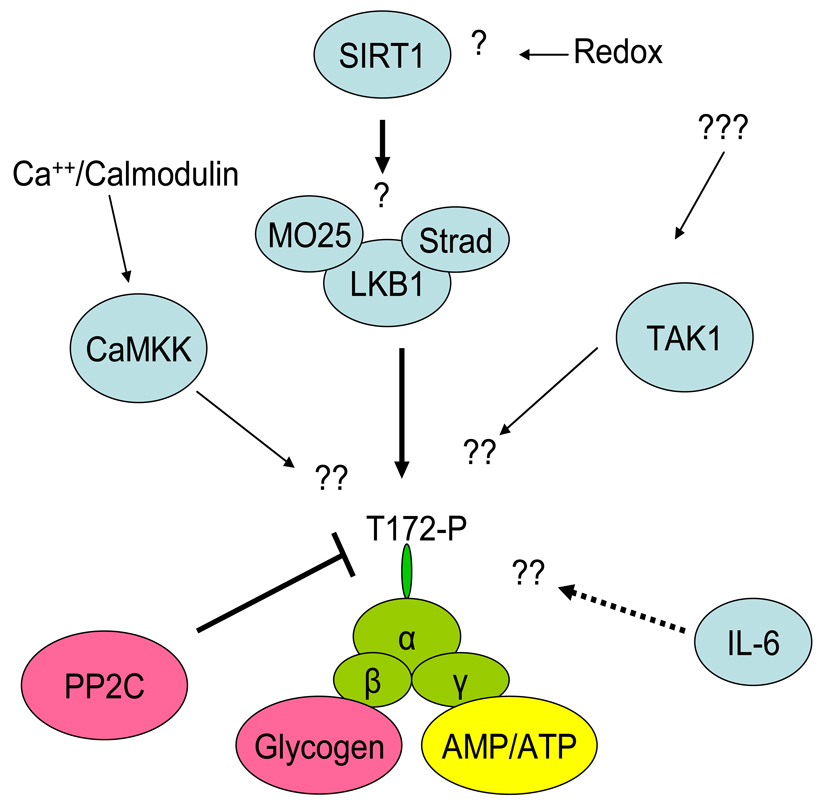
Figure 1.
Activation of AMPK in skeletal muscle during contraction/exercise. Although LKB1, the main kinase acting on α2 AMPK, is thought to be constitutively active, the possibility exists that SIRT1 may regulate LKB1 activity perhaps during prolonged exercise. In muscle, CaMKK may act as an AMPK kinase especially towards α1 AMPK during conditions in which AMP accumulation is limited. The role of TAK1 in muscle is uncertain. Binding of AMP to the γ subunit of AMPK makes AMPK a poor substrate for PP2C resulting in increased net phosphorylation of AMPK. IL-6 produced by contracting muscle may increase AMPK activity in this or adjacent muscle by an as yet unknown mechanism (broken line). The β subunit of AMPK has a glycogen binding domain and high glycogen levels in muscle inhibit AMPK activation during exercise.