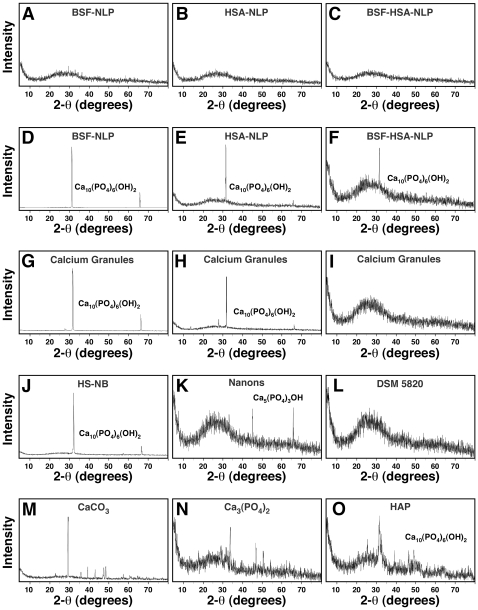
Figure 15. Powder X-ray diffraction spectra of protein-mineral nanoparticles reveal both amorphous and crystalline patterns.
Protein-mineral nanoparticles were obtained as described in Fig. 9, by diluting the proteins, separately (A and B) or together (C) into DMEM, followed by addition of the precipitating reagents CaCl2 and NaH2PO4 each to a final concentration of 0.3 mM and incubating the solutions in cell culture conditions for 1 month. The XRD spectra obtained for the protein-mineral nanoparticles obtained after 1 week of incubation represented mainly amorphous patterns as seen by the absence of diffraction peaks (A–C). Longer incubation of 1 month produced protein-mineral nanoparticles with crystalline peaks corresponding to HAP crystals (D–F, Ca10(PO4)6(OH)2). XRD spectra were also obtained for calcium granules that were prepared by adding either CaCl2 (G) or NaH2PO4 (H) into FBS or both CaCl2 and NaH2PO4 into HS (I), followed by sample preparation as described in the Materials and Methods . Peaks corresponding to Ca10(PO4)6(OH)2 were obtained for both calcium granules prepared in FBS (G and H) while the ones prepared in HS usually gave amorphous patterns (I). XRD spectra showing the presence of HAP crystals (J), a calcium phosphate compound (K, Ca5(PO4)3OH), or an amorphous pattern (L) were also acquired for NB cultured in 10% HS for 1 month (J, “HS-NB”) or in 10% FBS for 1 month (K, “Nanons”) or 1 week (L, “DSM 5820”). Commercial grades of CaCO3 (M), Ca3(PO4)2 (N), and HAP (O), used as controls, were diluted and washed in double-distilled water.