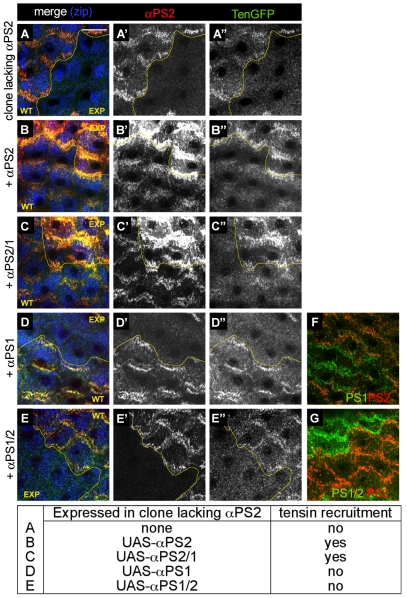
Fig. 8.
αPS2βPS is able to recruit tensin whereas αPS1βPS is not, and the specificity resides in the extracellular or transmembrane domain of αPS2. (A-G) Wild-type cells (WT) adjacent to experimentally perturbed clones of cells (EXP); a yellow line marks the interface. (A-A″) Cells lacking αPS2 (ifB4 mutant clones), revealed by absence of of αPS2 (red, A′) failed to recruit tensin-GFP (A″); the presence of stress fibres was shown by non-muscle myosin II (Zipper, Zip, blue in A). Re-expressing αPS2 (B-B″) or a chimera αPS2/1 with the cytoplasmic domains swapped (C-C″) in cells lacking αPS2 recruited tensin-GFP (note that αPS2 is `overexposed' in B′ and C′ because the level of re-expressed protein is elevated relative to wild type, and the level of αPS2 in the wild-type cells was used to set image levels). Conversely, αPS1 (D-D″) or the inverse chimera αPS1/2 (E-E″) failed to restore tensin-GFP recruitment. (F,G) Control images confirm the replacement of αPS2 with αPS1 or αPS1/2 (detected with the anti-αPS1 antibody) in similar clones to those in D and E. Scale bar: 20 μm.