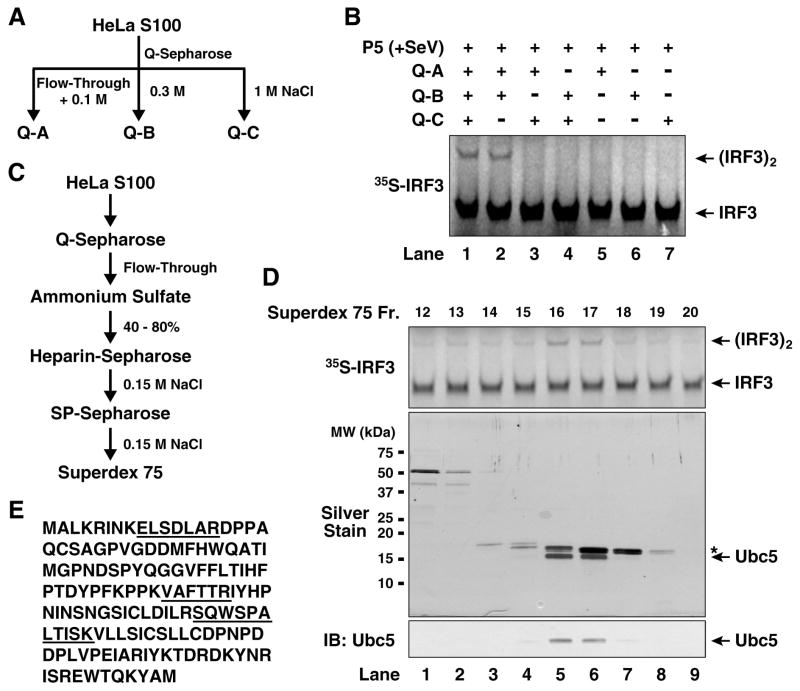
Figure 2. Identification of Ubc5 as an IRF3 activator.
(A) Diagram of initial fractionation of HeLa S100 on Q-Sepharose column. After loading the sample, the column was eluted step-wise with 0.1 M (Q-A; also contains the flow-through fraction), 0.3 M (Q-B) and 1.0 M NaCl (Q-C). (B) Reconstitution of IRF3 activation with Q-Sepharose fractions. In vitro assay for IRF3 activation was carried out as described in Figure 1, except that the cytosolic extracts were replaced with the indicated combinations of Q-A, Q-B and Q-C. (C) Scheme of biochemical fractionation of Q-A. (D) Identification of Ubc5 as the active component in Q-A. Aliquots of the fractions from Superdex-75 were tested for their ability to stimulate IRF3 dimerization in the presence of Q-B and virus-activated P5 (upper panel), and analyzed by silver staining (middle panel) and immunoblotting with a Ubc5 antibody (lower panel). The asterisk (*) in the silver-stained gel denotes a contaminating protein (cyclophilin A). (E) Amino acid sequence of Ubc5c. Peptide sequences identified by mass spectrometry are underlined.