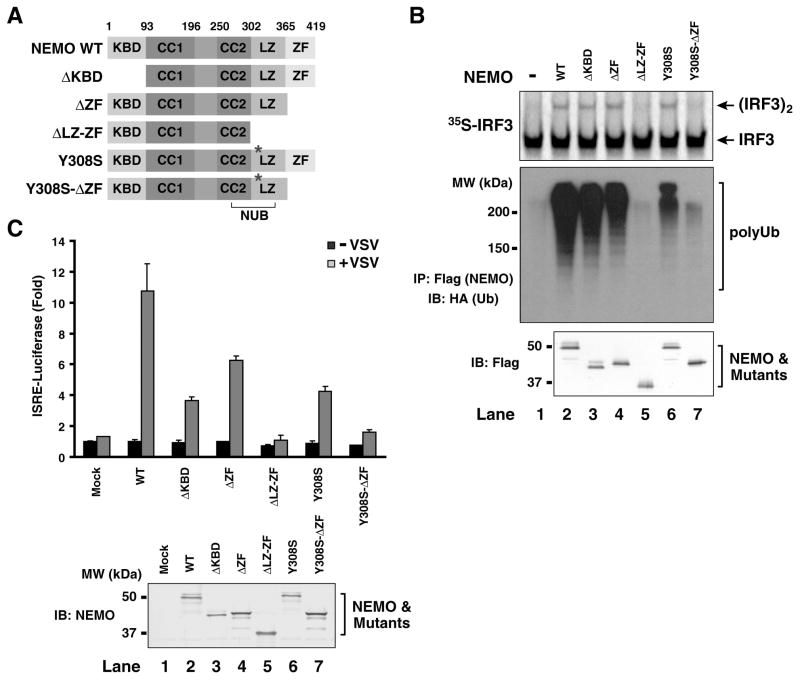
Figure 6. Ubiquitin-binding domains of NEMO are required for IRF3 activation.
(A) Diagrams of NEMO domains and its mutants. KBD, IKK-binding domain; CC1 and CC2, coiled-coil domain 1 and 2; LZ, leucine-zipper motif; ZF, zinc-finger domain; NUB: NEMO-ubiquitin binding. (B) Ubiquitin-binding of NEMO is required for IRF3 activation in vitro. Expression vectors for Flag-NEMO and its mutants as shown in (A) were transfected into HEK293T cells, then the proteins were purified using anti-Flag (M2) agarose. Equal amounts of the NEMO proteins (bottom panel) were incubated with cytosolic extracts from NEMO-deficient MEF cells together with the virus-activated mitochondrial fraction (P5). Dimerization of 35S-IRF3 was analyzed by native gel electrophoresis followed by autoradiography (upper panel). Aliquots of NEMO proteins were incubated with K63-linked HA-Ub chains and immunoprecipitated with anti-Flag agarose beads. Co-immunoprecipitated polyubiquitin chains were analyzed by immunoblotting (middle panel). (C) Ubiquitin-binding of NEMO is required for viral activation of IRF3. NEMO−/− MEF cells were transiently transfected with NEMO and its mutants together with the ISRE- luciferase reporter and a control plasmid (pRL-CMV). The cells were infected with VSV (ΔM51)-GFP for 14 hours before cell lysates were prepared for luciferase assays. The error bars represent variation ranges of duplicate experiments.