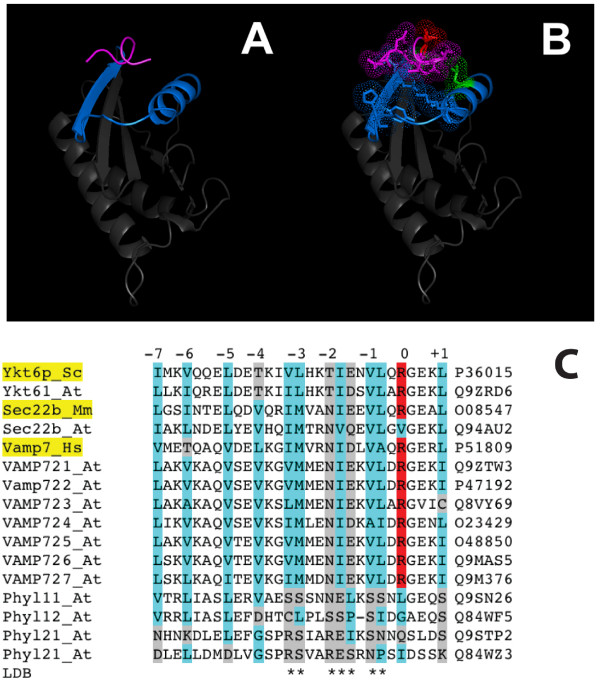
Figure 5.
Phyre (threading method) based prediction of intramolecular binding in a representative Phytolongin [UniProt: Q9SN26]. Panel A: a short motif from the PhyL region (magenta) is suggested to bind to the α1-β3 region (blue) of the LD (grey). Panel B: binding is likely based on polar side chains (LD, blue; PhyL motif, magenta); hydrophobic side chains from the LD are green and the only one from the PhyL region is red. Structural representations were obtained using PyMOL. The alignment in panel C is centered around the LD binding (LDB) motif of structurally solved longins (highlighted in yellow). Homologous Ykt6, Sec22b and VAMP7 family longins and the four Phytolongins from Arabidopsis thaliana are also included. The conserved Arg residue at the zero layer of the SNARE motif is highlighted in red. Hydrophobic or polar residues are highlighted in respectively cyan and grey in columns concerning the heptad repeat layers or the LDB. The putative LDB region of Phytolongins is clearly more polar than LDB of longins, and the Arg residue is not conserved. Instead, several hydrophobic layer positions are conserved with the Phytolongins. Conservation is not apparent in the CT half (not shown).