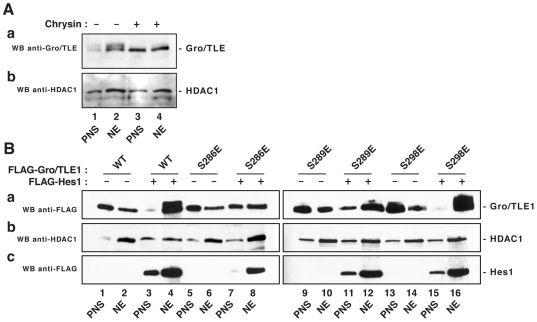
Figure 6. Effect of SP domain mutations on the association of Gro/TLE1 with chromatin.
(A) Pharmacological inhibition of protein kinase CK2. A subcellular fractionation procedure was performed to obtain postnuclear supernatants (PNS) and chromatin-enriched nuclear extracts (NE) from cells transfected with an Hes1-expression plasmid to promote the cofactor activated hyperphosphorylation of endogenous Gro/TLE proteins. Equivalent amounts of fractions were subjected to SDS-polyacrylamide gel electrophoresis (6% gel), followed by sequential Western blotting (WB) with anti-Gro/TLE (‘panTLE’) (a) and anti-HDAC1 (b) antibodies. Treatment with chrysin resulted in reduced nuclear retention of endogenous Gro/TLE, but not HDAC1, proteins. It also caused a decrease of the more slowly migrating, hyperphosphorylated Gro/TLE form(s), as previously reported (27). (B) Analysis of the chromatin association of Gro/TLE1 by subcellular fractionation. Postnuclear supernatant and chromatin-enriched nuclear extracts from cells transfected with the indicated combinations of FLAG-tagged proteins were subjected to SDS-polyacrylamide gel electrophoresis, followed by sequential Western blotting with anti-FLAG (a and c) and anti-HDAC1 (b) antibodies, as indicated. Both the hyperphosphorylation (readily detactable on 6% gels) and increased chromatin association of wild type Gro/TLE1 induced by Hes1 were impaired by the SP mutations S286E and S289E (cf. lanes 3 and 4 to lanes 7 and 8, 11 and 12), but not by the S298E mutation (cf. lanes 3 and 4 to lanes 15 and 16). No changes were observed in the strength of the nuclear association of Hes1 and HDAC1 (panels b and c). Hes1 was consistently expressed at slightly lower levels when co-expressed with the S286E mutant. Shown is a representative example of three separate experiments.