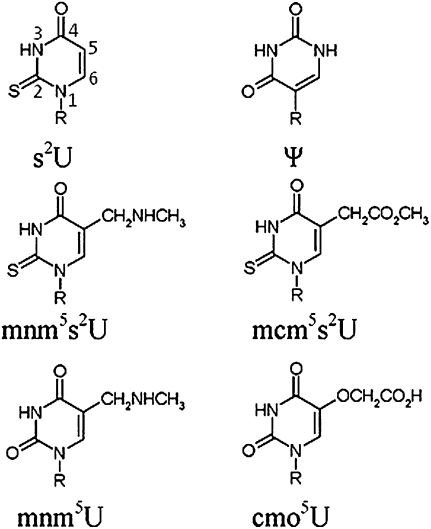
FIGURE 3.
Chemical structures of the modified nucleosides. Modified uridine chemical structures: 2-thiouridine (s2U), pseudouridine (Ψ), 5-methylaminomethyluridine (mnm5U), 5-methoxy-carbonylmethyluridine (mcm5s2U), 5-oxyacetic acid uridine (cmo5U), and 5-methylaminomethyl-2-thiouridine (mnm5s2U). R represents the ribose sugar attached to the base and 5′-phosphate. The first base demonstrates the standard uracil base numbering and can be applied throughout.