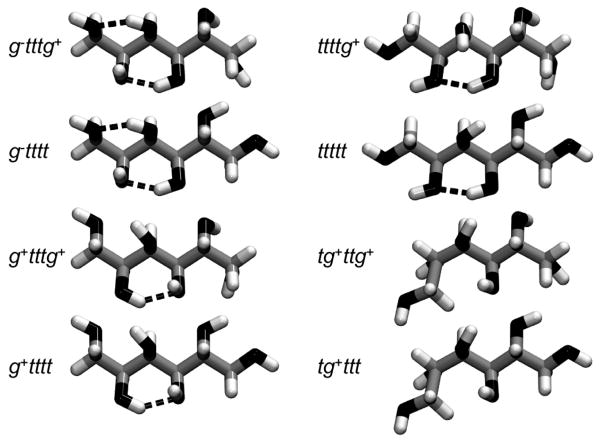
Figure 4.
The most populated conformations of sorbitol (the orientation of molecules is similar to that in Figure 2, with C-1 and C-6 carbons on the left and right extremities of molecules, respectively). The different conformations are labeled according to which minimum each dihedral angle belongs; i.e., ijklm denotes the conformation in which the dihedral angles φ1, φ2, φ3, φ4, and φ5 (defined in the text and in the caption of Figure 3) are found in minima i, j, k, l, and m, respectively. The conformations shown have been obtained from the minimization of a sorbitol molecule in vacuum with the steepest descent and conjugate gradient algorithms, while applying a constraint potential of 1000 kcal/mol on each dihedral angle φ. Hydrogen bonds between hydroxyl groups are represented by a dashed line (a hydrogen bond was assumed to exist if the O---O distance is less than 3.5 Å and the O–H---O bond angle is greater than 120°).