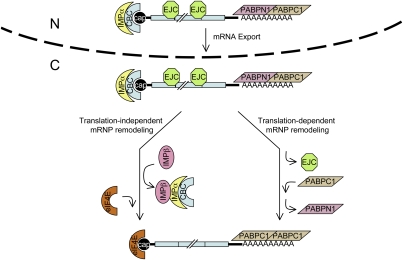
Figure 8.
Schematic of translation-induced remodeling and translation-independent, IMPβ-induced remodeling of the pioneer translation initiation complex. The pioneer translation initiation complex is exported from nuclei (N) to the cytoplasm (C) bound by (1) CBC at the 5′-cap; (2) EJCs, which contain eIF4AIII and NMD factors that include UPF3X situated upstream of exon–exon junctions, provided the mRNA underwent splicing; (3) PABPN1 and PABPC1 at the poly(A) tail; and (4) other initiation factors that are not shown, including eIF4G and eIF3. Notably, IMPα associates with CBC via the NLS of CBP80, and the analysis of unspliced pre-mRNA indicates that IMPα begins associating with CBC-bound transcripts prior to their splicing (Supplemental Fig. S4), possibly concomitantly with CBC binding to nascent transcripts. Data indicate that the pioneer round of translation removes the EJCs (Fig. 3; Supplemental Fig. S2) and promotes the replacement of PABPN1 by PABPC1 (Fig. 4; Supplemental Figs. S1, S2) but is inconsequential to the association of IMPα with the CBC (Fig. 5) or the replacement of CBC by eIF4E (Fig. 3). Instead, our findings suggest that the interaction of IMPβ with IMPα promotes the replacement of CBC by eIF4E (Figs. 6, 7), which depends on the RAN–GTP-mediated delivery of nuclear IMPβ to the cytoplasm and, subsequently, promotion of IMPβ binding to IMPα–CBC by the GTPase-activating protein RAN-GAP. The replacement of CBC by eIF4E is followed by the trafficking of IMPβ–IMPα–CBC to nuclei. Once in the nucleus, IMPβ dissociates from IMPα–CBC. This frees IMPα–CBC to bind to the 5′ cap of newly made transcripts so as to function once again in the nuclear export and cytoplasmic remodeling of mRNP.