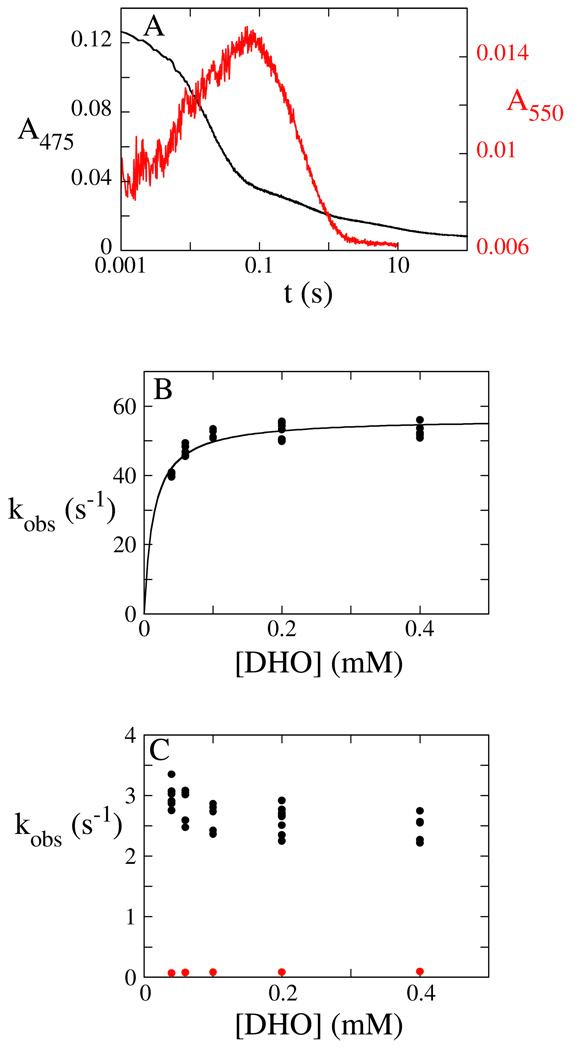
Figure 4.
Reductive Half-Reaction of the Phe115Leu Mutant DHOD at pH 10. (A) Enzyme (~9.5 µM after mixing) was mixed with DHO (400 µM after mixing) in an anaerobic stopped-flow experiment at 4 °C. The dead-time of the stopped-flow instrument was 1 ms. At 475 nm (black), the reaction occurs in three phases. The first (large) phase represents the chemical step. The second phase represents product release. At 550 nm (red), flavin reduction is seen as an increase in absorbance and product dissociation is seen as a decrease in absorbance. Reaction traces are shown on a log scale. (B) The observed rate constant of the first phase (flavin reduction) saturates with increasing DHO concentration, giving a reduction rate constant (kred) of 56.5 ± 0.9 s−1 and an estimate of the Kd,DHO of 14 ± 2 µM. (C) The observed rate constant of the second phase (black), OA release, decreases with increasing DHO concentration, giving a limiting value of 2.5 s−1. The observed rate constant of the final phase (red) is ~0.08 s−1 and is independent of DHO concentration.