Abstract
The U.S. active-duty military population may differ from the U.S. general population in its exposure to cancer risk factors and access to medical care. Yet, it is not known if cancer incidence rates differ between these two populations. We therefore compared the incidence of four cancers common in U.S. adults (lung, colorectum, prostate, and breast cancers) and two cancers more common in U.S. young adults (testicular and cervical cancers) in the military and general populations. Data from the Department of Defense's Automated Central Tumor Registry (ACTUR) and the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) nine cancer registries for the years 1990-2004 for persons aged 20-59 years were analyzed. Incidence rates were significantly lower in the military population for colorectal cancer in white men, lung cancer in white and black men and white women, and cervical cancer in black women. In contrast, incidence rates of breast and prostate cancers were significantly higher in the military among both whites and blacks. Incidence rates of testicular cancer did not differ between ACTUR and SEER. Although the numbers of diagnoses among military personnel were relatively small for temporal trend analysis, we found a more prominent increase in prostate cancer in ACTUR than in SEER. Overall, these results suggest that cancer patterns may differ between military and non-military populations. Further studies are needed to confirm these findings and explore contributing factors.
Keywords: Active duty, cancer, incidence, military, SEER
Introduction
The U.S. military population may differ from the U.S. general population in exposure to factors associated with cancer risk, such as physical fitness, smoking, alcohol consumption, diet, and sunlight exposure. Exposures associated with military deployments, such as immunizations and depleted uranium, may also influence cancer risk among military personnel. Yet, compared with the general population, the military population may be generally healthier and more likely to undergo cancer screening and surveillance because military members have free access to health care. Despite these potential differences, cancer incidence rates in the U.S. military and general populations have not been extensively compared.
The elucidation of differences in cancer incidence patterns between the military and general U.S. populations may lead to a better understanding of etiology and the development of preventive strategies for both populations. Researchers have often used data from cancer registries such as the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute to study demographic patterns and trends in incidence and generate study hypotheses (1-7).
Studies on cancer incidence rates in the U.S. military population are few. Using the data from the Department of Defense (DoD) Automated Central Tumor Registry (ACTUR) and the Defense Manpower Data Center, Thompson et al. found that the incidence of testicular cancer among active duty members of the military had increased over time (8). Yamane's study of ACTUR data from 1989 to 2002 for U.S. Air Force active-duty personnel found that cervical, prostate, and vulvar cancers were more frequent than expected, while bladder, brain, colorectal, oral squamous cell, and testicular cancers, as well as lymphomas, were less frequent than expected in comparison with national data (9). These two studies focused on either a specific cancer (8) or a specific military service (9). Therefore, the current study was conducted to gain a broader picture of cancer among military members by comparing incidence patterns of six cancers among all active-duty military personnel and the general U.S. populations.
Materials and Methods
This study was based on non-identifiable data and was approved by the Institutional Review Boards of United States Military Cancer Institute, Armed Forces Institute of Pathology, and the National Cancer Institute.
ACTUR was established in 1986 as the cancer database and clinical tracking system for the DoD. Military medical treatment facilities (MTFs) are required to report cancer data on all DoD beneficiaries, including active-duty military personnel and their family members, retired military personnel, and Reserve and National Guard personnel who are temporarily activated. For the current study, data on diagnoses from 1990 to 2004 were analyzed.
For this study, data analyses were confined to personnel on active military duty. Records for retired military personnel, reservists, National Guard personnel, and family members are less complete as they may get medical care outside the military system.
To reconcile duplicate records for the same patient so that only one summary record existed for each primary cancer, we used data consolidation procedures based on national and state cancer registry guidelines (10-12). The guidelines were used to determine multiple primary malignancies and to select the best information on diagnostic and demographic variables from the multiple records per person that exist in ACTUR.
The following items from the ACTUR database were used in the data analysis: primary cancer site, age at diagnosis, gender, and race. Diagnoses are classified using the International Classification of Diseases for Oncology (ICD-O-3)(13). The annual numbers of active-duty military personnel were used to calculate incidence rates. The numbers were obtained from the Defense Manpower Data Center, which maintains demographic and military data on personnel in all military services.
National comparison data were obtained from the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute(14). SEER collects and publishes cancer statistics from population-based cancer registries. For the current study, cancer rates for 1990 – 2004 were drawn from the SEER - 9 Registries Database (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah), covering approximately 10% of the US population
The age distribution of the active military population differs substantially from that of the general population in that there are no members of the military younger than 17 years old and few 60 years or older. As the small number of persons <20 years and 60+ years would generate statistically unstable rates, analyses were restricted to persons aged 20 to 59 years. Furthermore, within this age range, the active-duty military population is considerably younger than the general US population. To give more weight to the age groups with a large number of active-duty members, and thereby, generate more stable rates, the 1990-2004 military population was used as the standard population for age adjustment. Age-adjusted incidence rates, incidence rate ratios (IRRs), and their 95% confidence intervals (CIs) were calculated. The Tiwari method was used to estimate CIs. Because the military population was used as the standard population for these calculations, the absolute incidence rates in this study differ from those based on US census data.
We analyzed the incidence of six cancers (lung, colorectum, prostate, testis, breast, and cervix) by gender, year of diagnosis (1990-1994, 1995-1999, 2000-2004) and race (white, black). Small numbers of military patients precluded the examination of data among other racial or ethnic groups. Small numbers also precluded trend analyses of lung cancer and testicular cancer among black men, colorectum cancer and lung cancer among women, and cervical cancer among black women.
Results
Among the six cancers examined, the most common among active duty military personnel (the ACTUR population) was testicular cancer (N = 1,826), followed by prostate (N = 910), breast (N = 864), and colorectal (N = 738) cancers. In the SEER population, breast cancer (N = 107,601) among women was the most common cancer in this 20-59 year age group, followed by lung (N = 46,083), prostate (N = 42,751) and colorectal (N = 36,092) cancers (Table 1). These frequencies reflect the dramatic differences in the size and gender/age distribution of the two populations. Colorectal cancer incidence among white men was significantly lower in the ACTUR population than the SEER population (IRR = 0.83), but there were no differences among the other three race/gender groups. Lung cancer incidence was significantly lower in all groups of the ACTUR population compared to the SEER population except for black women (IRR = 0.58, 0.69, 0.35, 1.10 among white men, white women, black men, and black women, respectively). Cervical cancer incidence was significantly lower in the ACTUR compared to SEER among blacks (IRR = 0.43) but not among whites (IRR = 0.92).
Table 1.
Cancer incidence in the U.S. active-duty military population and the SEER program for breast, lung, prostate, colorectal, testicular, and cervical cancers, ages 20-59, 1990-2004, by race and gender.
| ACTUR1 | SEER2 | ACTUR:SEER | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Whites | Blacks | Whites | Blacks | Whites | Blacks | |||||||||||
| Cancer site | Count | Rate3 | Rate 95%CI4 | Count | Rate3 | Rate 95%CI4 | Count | Rate3 | Rate 95%CI4 | Count | Rate3 | Rate 95%CI4 | IRR5 | IRR 95%CI | IRR5 | IRR 95%CI |
| Men | ||||||||||||||||
| Colorectum | 513 | 3.49 | 3.19-3.80 | 146 | 4.89 | 4.06-5.83 | 17642 | 4.22 | 4.09-4.35 | 2693 | 5.31 | 4.95-5.68 | 0.83 | 0.75-0.91 | 0.92 | 0.75-1.11 |
| Lung | 277 | 1.85 | 1.63-2.08 | 70 | 2.49 | 1.91-3.20 | 20682 | 3.17 | 3.08-3.27 | 4957 | 7.21 | 6.83-7.60 | 0.58 | 0.51-0.66 | 0.35 | 0.26-0.45 |
| Prostate | 689 | 4.32 | 4.00-4.65 | 221 | 10.02 | 8.64-11.57 | 35175 | 2.03 | 1.99-2.08 | 7576 | 4.80 | 4.60-5.01 | 2.12 | 1.95-2.30 | 2.09 | 1.77-2.43 |
| Testis | 1756 | 12.63 | 12.05-13.23 | 70 | 1.97 | 1.54-2.50 | 9318 | 13.11 | 12.79-13.43 | 243 | 2.17 | 1.87-2.52 | 0.96 | 0.91-1.02 | 0.91 | 0.67-1.21 |
| Women | ||||||||||||||||
| Colorectum | 49 | 3.26 | 2.41-4.31 | 30 | 4.52 | 3.01-6.52 | 13051 | 3.41 | 3.29-3.53 | 2706 | 5.03 | 4.69-5.39 | 0.96 | 0.69-1.27 | 0.90 | 0.58-1.31 |
| Lung | 30 | 1.99 | 1.34-2.84 | 25 | 4.70 | 2.78-7.33 | 17413 | 2.89 | 2.80-2.99 | 3031 | 4.27 | 3.99-4.56 | 0.69 | 0.45-0.98 | 1.10 | 0.62-1.73 |
| Breast | 543 | 36.44 | 33.43-39.64 | 321 | 45.87 | 40.65-51.61 | 95058 | 30.62 | 30.29-30.96 | 12543 | 33.41 | 32.54-34.31 | 1.19 | 1.09-1.30 | 1.37 | 1.21-1.55 |
| Cervical | 113 | 7.31 | 6.02-8.80 | 30 | 3.42 | 2.30-4.90 | 9308 | 7.92 | 7.70-8.14 | 1732 | 7.97 | 7.47-8.49 | 0.92 | 0.75-1.11 | 0.43 | 0.28-0.62 |
ACTUR, Automated Central Tumor Registry.
SEER, Surveillance, Epidemiology and End Results.
Rates are per 100,000 person-years and age-adjusted to active-duty military population aged 20-59.
Confidence interval.
Incidence rate ratio.
In contrast to the lower rates of colorectal, lung and cervical cancers among some military personnel, the incidence rates of prostate and breast cancers were significantly higher in the ACTUR population (prostate cancer IRR = 2.12 and 2.09, and breast cancer IRR = 1.19 and 1.37 among whites and blacks, respectively). Testicular cancer rates did not differ between the ACTUR and SEER populations (IRR = 0.96 and 0.91 among whites and blacks).
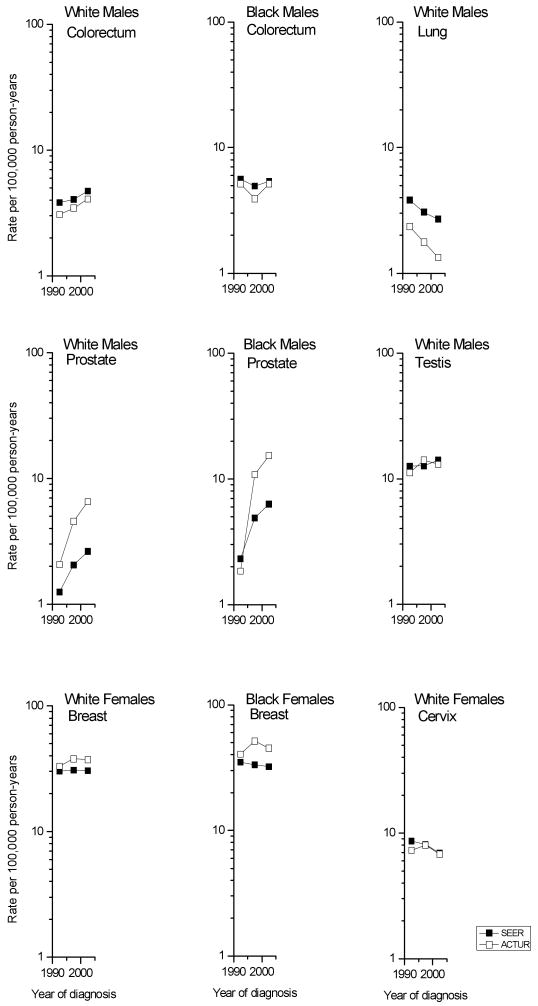
Figure 1 and Table 2 show the temporal trends in race-and gender-specific age-adjusted incidence rates among the ACTUR and SEER populations from 1990-94 to 2000-04. Among men, colorectal cancer incidence increased significantly among both ACTUR and SEER whites (33% and 23%, respectively), but not blacks. In contrast, lung cancer rates decreased 43% and 30% among ACTUR and SEER white men. Prostate cancer rates increased significantly among both whites and blacks in both populations. The increases in both racial groups appeared more prominent in ACTUR than in SEER: rates doubled among SEER whites, but tripled among ACTUR whites. Among blacks, SEER rates rose more than doubled, while among ACTUR blacks, rates increased more than eight-fold. Testicular cancer rates rose non-significantly among white men in both populations.
Figure 1.
Trends in cancer incidence rates among active-duty members and in the SEER program, 1990-94 to 2000-04 (rates age-adjusted using the active duty military population aged 20-59). ACTUR, Automated Central Tumor Registry; SEER, Surveillance, Epidemiology and End Results.
Table 2.
Incidence in the U.S. active-duty military population and the SEER program for breast, lung, prostate, colorectal, testicular, and cervical cancers by race and gender, ages 20-59: comparison of 2000-2004 to 1990-1994.
| 1990-1994 | 2000-2004 | ||||
|---|---|---|---|---|---|
| Cancer site | Count | Rate3 (95% CI4) | Count | Rate3 (95% CI4) | IRR5 (95%CI3) |
| Colorectum | |||||
| White males | |||||
| ACTUR1 | 171 | 3.08 (2.63-3.57) | 182 | 4.09 (3.51-4.74) | 1.33 (1.07-1.65) |
| SEER2 | 5099 | 3.84 (3.64-4.05) | 6802 | 4.74 (4.49-4.99) | 1.23 (1.14-1.33) |
| Black males | |||||
| ACTUR | 58 | 5.17 (3.82-6.84) | 47 | 5.16 (3.74-6.95) | 1.00 (0.65-1.54) |
| SEER | 727 | 5.62 (4.96-6.34) | 1105 | 5.40 (4.82-6.03) | 0.96 (0.81-1.14) |
| Lung | |||||
| White males | |||||
| ACTUR | 130 | 2.35 (1.96-2.79) | 63 | 1.34 (1.02-1.72) | 0.57 (0.41-0.78) |
| SEER | 7137 | 3.83 (3.65-4.02) | 6672 | 2.70 (2.54-2.86) | 0.70 (0.65-0.76) |
| Prostate | |||||
| White males | |||||
| ACTUR | 112 | 2.07 (1.70-2.49) | 352 | 6.55 (5.87-7.28) | 3.17 (2.55-3.96) |
| SEER | 6877 | 1.25 (1.18-1.31) | 16704 | 2.63 (2.53-2.72) | 2.11 (1.98-2.25) |
| Black males | |||||
| ACTUR | 11 | 1.85 (0.89-3.31) | 137 | 15.39 (12.81-18.33) | 8.33 (4.51-17.80) |
| SEER | 1258 | 2.32 (2.07-2.61) | 3824 | 6.33 (5.95-6.75) | 2.73 (2.39-3.11) |
| Testis | |||||
| White males | |||||
| ACTUR | 634 | 11.18 (10.33-12.09) | 515 | 13.09 (11.98-14.28) | 1.17 (1.04-1.32) |
| SEER | 2937 | 12.57 (12.04-13.12) | 3327 | 14.05 (13.48-14.64) | 1.12 (1.05-1.19) |
| Breast | |||||
| White females | |||||
| ACTUR | 164 | 33.23 (28.19-38.91) | 190 | 37.31 (32.07-43.13) | 1.12 (0.90-1.40) |
| SEER | 27067 | 30.43 (29.87-31.00) | 35275 | 30.68 (30.10-31.28) | 1.01 (0.98-1.04) |
| Black females | |||||
| ACTUR | 76 | 40.54 (26.13-59.75) | 122 | 45.12 (37.38-54.03) | 1.11 (0.74-1.80) |
| SEER | 3448 | 34.96 (33.34-36.63) | 4862 | 32.32 (30.88-33.81) | 0.92 (0.87-0.99) |
| Cervix | |||||
| White females | |||||
| ACTUR | 43 | 7.29 (5.25-9.88) | 30 | 6.80 (4.57-9.70) | 0.93 (0.56-1.52) |
| SEER | 3254 | 8.67 (8.28-9.07) | 2830 | 6.94 (6.58-7.30) | 0.80 (0.75-0.86) |
ACTUR, Automated Central Tumor Registry.
SEER, Surveillance, Epidemiology and End Results.
Rates are per 100,000 person-years and age-adjusted to active-duty military population.
Confidence interval.
Incidence rate ratio.
Among women, breast cancer rates did not change significantly in either population except among SEER blacks (IRR = 0.92). Cervical cancer rates among white women declined non-significantly in ACTUR (IRR = 0.93) and significantly in SEER (IRR = 0.80).
Discussion
Our study found differences in cancer incidence rates between military personnel and the general population. Rates were lower among military personnel than the general population for colorectal, lung, and cervical cancers. However, the differences in rates between the two populations were significant only for colorectal cancer among white males and cervical cancer among black females. Rates were significantly higher for prostate and breast cancers, and the rates of prostate cancer over time rose more rapidly among military personnel. There were no significant differences between the populations in the rates of testicular cancer.
It is unclear why white men in the military would have lower colorectal cancer incidence than other white men, though several factors may be related to the difference. Men in the military are a selected population as individuals with certain diseases or conditions are not eligible for military service. For example, military personnel may be less likely to be obese or to have familial polyposis. Individuals who enter the military are more physically active due to the fitness standards required for entry (9). Once in the military, servicemen might maintain healthier lifestyles than men in the general population. For instance, military personnel are generally engaged in more rigorous physical activities than their civilian counterparts as they must pass the military physical fitness tests and meet the military weight standards (http://www.military.com/military-fitness/fitness-test-prep/physical-fitness-test-standards and http://www.apft.net/). In addition, military personnel are granted free access to medical care and cancer screening services. As a result, precancerous lesions such as colonic polyps may be more likely to be detected and treated early in the military population (15-17), thereby potentially reducing the risk of colorectal cancer. These differences between the military and general populations were not significant for women and black men, although the same direction in IRR was observed. While this might be related to a relatively small number of patients for these groups, further research is needed to understand whether the racial/gender differences exist.
The significantly lower risk of cervical cancer among servicewomen vs. non-servicewomen might be related to greater access to medical care and cancer screening services in the military. Cervical cancer screening can result in the detection of pre-cancerous lesions and the treatment of these lesions may lower cervical cancer incidence rates. In the general population, black women are more likely than white women to have a low family income and a lack of usual source of medical care; factors that are associated with a lower rate of cervical cancer screening (18, 19). In the military population, access to medical care is equal between black and white women. This might have produced the larger differences between the two populations in incidence of cervical cancer in black women. Further research is needed on why the incidence rate is lower in black women than white women in the military.
Lung cancer rates were significantly lower in the military among all groups except black women. Cigarette smoking is the single most important risk factor for lung cancer. In the past, the prevalence of smoking in the military, particularly among junior enlisted military personnel (20, 21), exceeded that in the general population (22, 23). Therefore, the lower rate of lung cancer in military personnel is an unanticipated finding. It is possible, however, that smoking patterns, which influence the risk of lung cancer, differ among military and non-military personnel. While most smokers in the general population begin smoking in their teens, adult-onset smoking is a phenomenon seen in military populations (24). It is also possible that, due to the emphasis on physical activity in the military, servicepersons smoke fewer cigarettes than their counterparts not in the military.
The prevalence of smoking in the U.S. has been declining, especially among males, and lung cancer rates have been decreasing, especially among the younger and middle age groups (25). Among military members, cigarette smoking dropped sharply from 1980 to 1998 while it increased somewhat afterwards (26). The lower rates in the military compared to SEER are welcome observations.
Prostate cancer rates in the military were twice those in the general population, and breast cancer rates were 20-40% higher. These differences may be related to free access to medical care for the military population. Military members may have more frequent visits to the doctor and thus are more likely to undergo breast and prostate cancer screening (27, 28). Several studies have now confirmed that cancer screening is associated with increases in breast and prostate cancer incidence rates (29-31).
In addition to the potential differences in screening practices between the military and general populations, variations in some risk factors may have contributed to the higher breast and prostate cancer rates in the military. With respect to breast cancer, military women may differ from those in the general population in reproductive history such as age at first birth, parity, and use of contraceptives. Military women may be more likely to use oral contraceptive pills because of a need or desire for anovulatory cycles and the easier access to prescription drugs. As shown in our recent analysis, 34% of active-duty women and 29% of women in the general population used OC pills in the preceding twelve months. Oral contraceptive pill use has been demonstrated to increase the risk of breast cancer, particularly in younger women (32, 33). Military women are also more likely to be engaged in industrial jobs than females in the general population and hence potentially more likely to be exposed to chemicals that may be related to breast cancer (34). A study in military women showed that those aged 34 or younger had higher age-specific incidence rates of breast cancer than women in the general population and the incidence was higher among military women with a moderate to high exposure to volatile organic chemicals than those with low or no exposure (34). Our findings of higher breast cancer rates in military women are consistent with those from this study.
In regard to prostate cancer, although the results have been inconsistent, depleted uranium (the material used in armor penetrators) has been suggested to increase the risk of prostate cancer (35, 36). Because military personnel are more likely to be exposed to depleted uranium, these factors may have contributed to the increased risk of prostate cancer in military members, although most of the increased rates and more dramatic increase over time in rates in military personnel might be attributed to screening in the population.
A number of factors may affect the comparability of the ACTUR and SEER databases. First, the two databases may differ in completeness of reporting. While cancer reporting is required by the Department of Defense, and regular training is provided to cancer registrars, some military treatment facilities do not have American College of Surgeons approved cancer programs. Some small clinics and hospitals may not have dedicated cancer registrars. In addition, some military personnel may be diagnosed and treated outside military treatment facilities through spouses' health insurance. Nevertheless, as long as military personnel are subsequently seen in a military treatment facility, which is usually true, they are included in the ACTUR data. These suggest that underreporting of cancer in ACTUR is of potential concern. However, the higher (rather than lower) breast and prostate rates in the military suggest that other factors beyond reporting are related to the observed differences between the two populations. Second, data consolidation procedures may vary between our analysis of the ACTUR dataset and SEER as no shared standards for case consolidation have been developed to date. Nevertheless, it is unlikely that data consolidation differences are substantial enough to account for the observed large differences in incidence rates of certain cancers such as prostate cancer. Our findings of similarities and differences in incidence rates between military personnel and the general population according to cancer, race, gender, and over time suggest that further research on risk factors and cancer screening practices in the military are warranted.
Acknowledgments
This research was supported by the United States Military Cancer Institute via the Uniformed Services University of the Health Sciences under the auspices of the Henry M. Jackson Foundation for the Advancement of Military Medicine and by the Division of Cancer Epidemiology and Genetics (DCEG), National Cancer Institute. The authors thank Ms. Annette Anderson of the Armed Forces Institute of Pathology for her help in obtaining the ACTUR data; Mr. William Mahr and Ms. Anne Dimke of United States Military Cancer Institute for their administrative support and help; Dr. Sally Bushhouse of Minnesota Cancer Surveillance System for providing the useful MN-PATRL document; John Lahey of IMS, Inc. and David Check of DCEG for figure development; Dr. Benedict Diniega, Col. John Kugler, and CAPT. David Arday of the Department of Defense Health Affairs for their insightful comments on the initial work on this topic; Dr. Joseph F. Fraumeni, Jr. and Dr. Robert N. Hoover of DCEG, and CDR. Kimberley Marshall, and CIV Thomas Williams of Tricare Management Activity for their encouragement and support for this collaborative project.
Footnotes
The opinions and assertions contained in this article represent the private views of the authors and do not reflect the official views of the U.S. Departments of the Army, Navy, or Defense, National Cancer Institute, or U.S. Government.
References
- 1.Hsing AW, Devesa SS. Trends and patterns of prostate cancer: what do they suggest? Epidemiol Rev. 2001;23:3–13. doi: 10.1093/oxfordjournals.epirev.a000792. [DOI] [PubMed] [Google Scholar]
- 2.McGlynn KA, Devesa SS, Sigurdson AJ, et al. Trends in the incidence of testicular germ cell tumors in the United States. Cancer. 2003;97:63–70. doi: 10.1002/cncr.11054. [DOI] [PubMed] [Google Scholar]
- 3.Wang SS, Sherman ME, Hildesheim A, Lacey JV, Jr, Devesa S. Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the United States for 1976-2000. Cancer. 2004;100:1035–44. doi: 10.1002/cncr.20064. [DOI] [PubMed] [Google Scholar]
- 4.Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005;117:294–9. doi: 10.1002/ijc.21183. [DOI] [PubMed] [Google Scholar]
- 5.Jatoi I, Anderson WF, Rao SR, Devesa SS. Breast cancer trends among black and white women in the United States. J Clin Oncol. 2005;23:7836–41. doi: 10.1200/JCO.2004.01.0421. [DOI] [PubMed] [Google Scholar]
- 6.Irby K, Anderson WF, Henson DE, Devesa SS. Emerging and widening colorectal carcinoma disparities between Blacks and Whites in the United States (1975-2002) Cancer Epidemiol Biomarkers Prev. 2006;15:792–7. doi: 10.1158/1055-9965.EPI-05-0879. [DOI] [PubMed] [Google Scholar]
- 7.Shah MN, Devesa SS, Zhu K, McGlynn KA. Trends in testicular germ cell tumours by ethnic group in the United States. Int J Androl. 2007;30:206–13. doi: 10.1111/j.1365-2605.2007.00795.x. discussion 13-4. [DOI] [PubMed] [Google Scholar]
- 8.Thompson IM, Optenberg S, Byers R, Dove M. Increased incidence of testicular cancer in active duty members of the Department of Defense. Urology. 1999;53:806–7. doi: 10.1016/s0090-4295(98)00609-8. [DOI] [PubMed] [Google Scholar]
- 9.Yamane GK. Cancer incidence in the U.S. Air Force: 1989-2002. Aviat Space Environ Med. 2006;77:789–94. [PubMed] [Google Scholar]
- 10.NAACCR. ATL site pairs table. ( http://www.naaccr.org/filesystem/other/ATL_Sitepairs_Table.xls) [PubMed]
- 11.Multiple and Histology Coding Rules. NCI SEER Program. 2007 [Google Scholar]
- 12.MCSS. MN-PATRL: Technical Specifications for Automated Record Consolidation. Minnesota Department of Health, 1997-2003 [Google Scholar]
- 13.Fritz A, Jack A, Shanmugaratnam K, et al. International Classification of Disease for Oncology. 3rd. Geneva: World Health Organization; 2000. [Google Scholar]
- 14.Ries L, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2005. National Cancer Institute; 2008. [Google Scholar]
- 15.Brawer MK. Prostatic intraepithelial neoplasia: an overview. Rev Urol. 2005;7 3:S11–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Schoenfield L, Jones JS, Zippe CD, et al. The incidence of high-grade prostatic intraepithelial neoplasia and atypical glands suspicious for carcinoma on first-time saturation needle biopsy, and the subsequent risk of cancer. BJU Int. 2007;99:770–4. doi: 10.1111/j.1464-410X.2006.06728.x. [DOI] [PubMed] [Google Scholar]
- 17.Giovannicci E, Wu K. Cancers of the colon and rectum. In: Schottenfeld D, Fraumeni JFJ, editors. Cancer Epidemiology and prevention. Oxford; Oxford University Press; 2006. pp. 809–29. [Google Scholar]
- 18.Hewitt M, Devesa SS, Breen N. Cervical cancer screening among U.S. women: analyses of the 2000 National Health Interview Survey. Prev Med. 2004;39:270–8. doi: 10.1016/j.ypmed.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 19.Peterson N, Murff H, Cui Y, Hargreaves M, Fowke J. Papanicolaou testing among women in the Southern United States. J Womens Health. 2008;17:939–46. doi: 10.1089/jwh.2007.0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunradi C, Moore R, Ames G. Contribution of occupational factors to current smoking among active-duty U.S. Navy careerists. Nicotine Tob Res. 2008;10:429–37. doi: 10.1080/14622200801889002. [DOI] [PubMed] [Google Scholar]
- 21.Peterson AL, Severson HH, Andrews JA, et al. Smokeless tobacco use in military personnel. Mil Med. 2007;172:1300–5. doi: 10.7205/milmed.172.12.1300. [DOI] [PubMed] [Google Scholar]
- 22.Chisick M, Poindexter F, York A. Comparing tobacco use among incoming recuits and military personnel on active duty in the United States. Tob Control. 1998;7:219–21. doi: 10.1136/tc.7.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bray RM, Marsden ME, Peterson MR. Standardized comparisons of the use of alcohol, drugs, and cigarettes among military personnel and civilians. Am J Public Health. 1991;81:865–9. doi: 10.2105/ajph.81.7.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haddock CK, Lando HA, Pyle SA, et al. Prediction of adult-onset smoking initiation among U.S. Air force recruits using the pierce susceptibility questionnaire. Am J Prev Med. 2005;28:424–9. doi: 10.1016/j.amepre.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Jemal A, Cokkinides VE, Shafey O, Thun MJ. Lung cancer trends in young adults: an early indicator of progress in tobacco control (United States) Cancer Causes Control. 2003;14:579–85. doi: 10.1023/a:1024891201329. [DOI] [PubMed] [Google Scholar]
- 26.Bray RM, Hourani LL. Substance use trends among active duty military personnel: findings from the United States Department of Defense Health Related Behavior Surveys, 1980-2005. Addiction. 2007;102:1092–101. doi: 10.1111/j.1360-0443.2007.01841.x. [DOI] [PubMed] [Google Scholar]
- 27.Ross LE, Berkowitz Z, Ekwueme DU. Use of the prostate-specific antigen test among U.S. men: findings from the 2005 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2008;17:636–44. doi: 10.1158/1055-9965.EPI-07-2709. [DOI] [PubMed] [Google Scholar]
- 28.Breast cancer screening in the military health system (MHS): a national quality management program special study. ACS Federal Healthcare, Inc.; 2002. [Google Scholar]
- 29.Collin SM, Martin RM, Metcalfe C, et al. Prostate-cancer mortality in the USA and UK in 1975-2004: an ecological study. Lancet Oncol. 2008;9:445–52. doi: 10.1016/S1470-2045(08)70104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lantz PM, Remington PL, Newcomb PA. Mammography screening and increased incidence of breast cancer in Wisconsin. J Natl Cancer Inst. 1991;83:1540–6. doi: 10.1093/jnci/83.21.1540. [DOI] [PubMed] [Google Scholar]
- 31.Jonsson H, Johansson R, Lenner P. Increased incidence of invasive breast cancer after the introduction of service screening with mammography in Sweden. Int J Cancer. 2005;117:842–7. doi: 10.1002/ijc.21228. [DOI] [PubMed] [Google Scholar]
- 32.Pymar HC, Creinin MD. The risks of oral contraceptive pills. Semin Reprod Med. 2001;19:305–12. doi: 10.1055/s-2001-18638. [DOI] [PubMed] [Google Scholar]
- 33.Yankaskas BC. Epidemiology of breast cancer in young women. Breast Dis. 2005;23:3–8. doi: 10.3233/bd-2006-23102. [DOI] [PubMed] [Google Scholar]
- 34.Rennix CP, Quinn MM, Amoroso PJ, Eisen EA, Wegman DH. Risk of breast cancer among enlisted Army women occupationally exposed to volatile organic compounds. Am J Ind Med. 2005;48:157–67. doi: 10.1002/ajim.20201. [DOI] [PubMed] [Google Scholar]
- 35.Ritz B. Cancer mortality among workers exposed to chemicals during uranium processing. J Occup Environ Med. 1999;41:556–66. doi: 10.1097/00043764-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Axelson O, Forastiere F. Radon as a risk factor for extra-pulmonary tumours. Med Oncol Tumor Pharmacother. 1993;10:167–7. doi: 10.1007/BF02989665. [DOI] [PubMed] [Google Scholar]