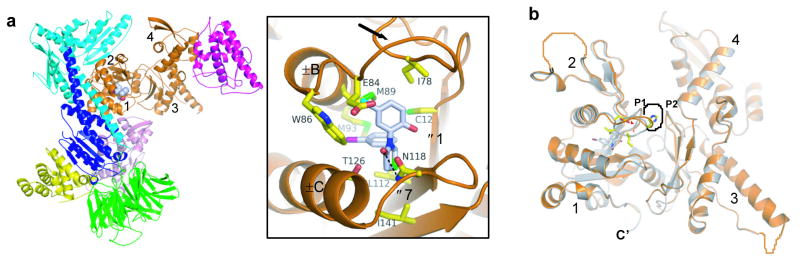
Figure 4.
Crystal structure of BtArp2/3 complex with bound CK548. The color coding is the same as in Figure 3. a, Ribbon diagram with detail of binding pocket. Black arrow marks the loop (residues 76–85) connecting β6 (73–75) and αB (86–98) in subdomain 1 of Arp3, which flips up to accommodate inhibitor binding. b, Ribbon diagrams of Arp3 with bound CK-548 (orange) overlaid onto the apo-BtArp2/3 structure (1K8K.pdb, grey). Black dotted line indicates the binding pocket for ATP or ADP. Orange dotted lines indicate disordered regions of the structure. Small red arrowheads indicate alternative conformations of the sensor loop. CK-548 is shown in stick representation with grey carbon atoms and select residues in the sensor loop from the 1K8K structure are shown as sticks with yellow carbon atoms.