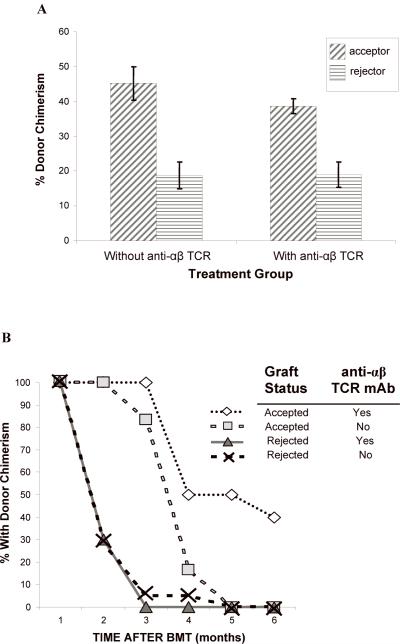
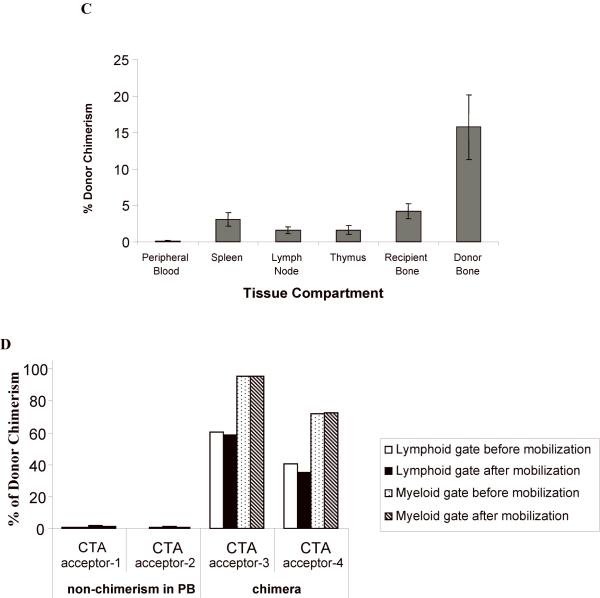
Figure 4. (A, B) The level of donor chimerism is predictive of flap acceptance.
Animals were followed for at least five months after CTA graft placement to monitor chimerism status and graft acceptance. There was a higher percentage donor chimerism at 1 month in CTA acceptors vs. rejectors in both treatment groups (A). PB donor chimerism was monitored monthly up to six months (B). The percent of animals that retained engraftment was defined as those animals that exhibited donor lymphoid chimerism above 1% at each time point. (C) Compartment chimerism in flap acceptors. Five rats that had accepted their heterotopic osteomyocutaneous flap for a minimum of six months underwent hematolymphopoietic compartment chimerism testing and multilineage analysis. The PB, spleen, thymus, mesenteric lymph nodes, donor bone, and recipient bone (contralateral femur) were harvested (> 6 months after CTA). One million cells were stained with the donor MHC class I marker: anti-RT1Aabl-FITC. The percent donor chimerism is shown as the percentage of donor cells in the lymphoid gate in the different tissue compartments. (D) Mobilization does not change chimerism in PB in long-term acceptors. Two long-term acceptors with PB donor chimerism and two long-term acceptors without PB donor chimerism received FL treatment (200 μg/kg) for 10 days and G-CSF (150 μg/kg) for 7 days (57). The donor cells were enumerated by staining of anti-donor ACI MHC class-I (anti-RT1Aabl FITC) 24 hours after completing the FL and G-CSF doses. The percentage of donor cells was analyzed in lymphoid or myeloid gates.