Abstract
Satellite cells, originating in the embryonic dermamyotome, reside beneath the myofibre of mature adult skeletal muscle and constitute the tissue-specific stem cell population. Recent advances following the identification of markers for these cells (including Pax7, Myf5, c-Met and CD34) (CD, cluster of differentiation; c-Met, mesenchymal epithelial transition factor) have led to a greater understanding of the role played by satellite cells in the regeneration of new skeletal muscle during growth and following injury. In response to muscle damage, satellite cells harbour the ability both to form myogenic precursors and to self-renew to repopulate the stem cell niche following myofibre damage. More recently, other stem cell populations including bone marrow stem cells, skeletal muscle side population cells and mesoangioblasts have also been shown to have myogenic potential in culture, and to be able to form skeletal muscle myofibres in vivo and engraft into the satellite cell niche. These cell types, along with satellite cells, have shown potential when used as a therapy for skeletal muscle wasting disorders where the intrinsic stem cell population is genetically unable to repair non-functioning muscle tissue. Accurate understanding of the mechanisms controlling satellite cell lineage progression and self-renewal as well as the recruitment of other stem cell types towards the myogenic lineage is crucial if we are to exploit the power of these cells in combating myopathic conditions. Here we highlight the origin, molecular regulation and therapeutic potential of all the major cell types capable of undergoing myogenic differentiation and discuss their potential therapeutic application.
Keywords: bone marrow, mesoangioblast, pericyte, satellite cell, side population, skeletal muscle, therapy
Introduction
Skeletal muscle is a highly dynamic tissue that is capable of regeneration following exercise-, immobilization- or chemically-induced damage. This regenerative capacity is due to the presence of a tissue-specific population of myogenic stem cells termed satellite cells. The satellite cell was first described by Mauro (1961) and is so-called due to its peripheral location on the skeletal muscle myofibre where it lies between the sarcolemma of the myofibre cell and its surrounding basal lamina (Fig. 1). Initial studies first suggested that the satellite cell may be capable of producing new myoblasts from which growing or damaged myofibres could form. However, lack of robust experimental procedures and tissue-specific markers for these cells prevented any further investigation. More recently, the identification of markers of satellite cells such as the transcription factors Pax7 and Myf5 along with the cell surface markers M-cadherin and CD34 in murine muscle have enabled investigators to show that the satellite cell is a major source of skeletal myoblasts that re-populate damaged and growing post-natal skeletal muscle as well as forming new myofibres (Beauchamp et al. 2000; Seale et al. 2000; Collins et al. 2005, see Table 1).
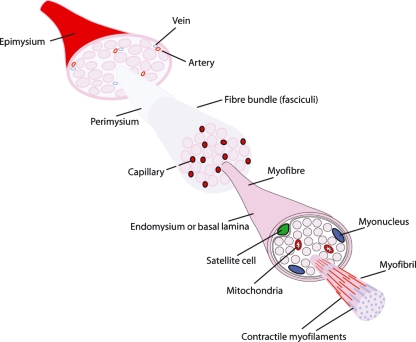
Fig. 1.
Satellite cell location within skeletal muscle structure. Individual muscle bellies, surrounded by an epimysium, are composed of bundles of myofibres, each separated by the perimysium and well supplied with blood vessels. Fibre bundles are each surrounded by a fascicle and infiltrated with a large capillary network contained within the connective tissue layer. Individual myofibre syncytiums, surrounded by an endomysium or basal lamina, are composed of multiple myonuclei, mitochondria and myofibrils that contain the contractile myofilament proteins. Satellite cells (green) lie between the syncytium and the basal lamina of the myofibre.
Table 1.
Summary of separate myogenic stem cell population markers and regenerative potentials
| Cell type | Cell markers | In vitro myotube formation | In vivo myofibre formation | Satellite cell niche engraftment | mdx dystrophin restoral |
|---|---|---|---|---|---|
| Satellite cell | Pax7, Myf5, CD34, c-Met, | Yes | Yes | Yes | Yes |
| HSC | c-Kit, Sca1, CD45 | Yes | Yes | No | Yes |
| HSC SP | c-Kit, Sca1, CD45 | Yes | Yes | Yes | Yes |
| Skeletal muscle SP | Sca1, Syndecan4, Pax3? | Yes | Yes | Yes | Yes |
| Muscle-derived stem cells | CD34, Bcl2 | Yes | Yes | Unknown | Yes |
| Mesoangioblast | CD34, c-Kit, Flk1 | Yes | Yes | Unknown | Yes* |
| Pericyte | α-SMA | Yes | Yes | Yes | Yes |
| Adipose tissue-derived mesenchymal stem cells | CD13, CD44, CD73, CD90 | Yes | Yes | Unknown | Yes |
Mesoangioblasts successfully restored α-sarcoglycan expression following delivery into α-sarcoglycan−/− mice (Sampaolesi et al., 2003).
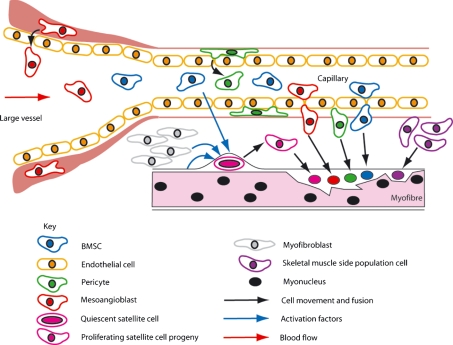
However, a number of studies have identified other cell types that show the capability of forming differentiated skeletal muscle tissue in vivo, and more importantly, some have been proposed to populate the satellite cell niche, thus forming new muscle stem cells. Differing stem cell types have been shown to be able to undergo these processes including skeletal muscle side population cells, bone marrow-derived haematopoietic lineages, mesoangioblast and pericyte endothelial precursor cells of blood vessel walls and, quite recently, brown fat precursor cells (Gussoni et al. 1999; Sampaolesi et al. 2006; Dellavalle et al. 2007; Seale et al. 2008; Fig. 2). Many of these cell types are able to carry out satellite cell niche engraftment and myofibre formation following systemic delivery and thus show potential for therapeutic use. However, the efficacy of many of these cells to incorporate into damaged muscle has yet to equal that of the intrinsic satellite cell population. In this review we first discuss the key features of skeletal muscle satellite cell biology and some of the key signalling pathways that are involved in the activation, proliferation and differentiation of these cells into muscle. Subsequently, we focus on the other major cell lineages that are also capable of forming skeletal muscle tissue in vivo and discuss their origin, properties, tissue developmental repertoire and potential benefits for therapeutic use.
Fig. 2.
Schematic depicting the major cell types of the body able to engraft into regenerating skeletal muscle.
Satellite cells
Skeletal muscle stem cells, named satellite cells due to their peripheral, sub-laminar position, were first discovered through electron microscopic analysis of skeletal muscle myofibres (Mauro, 1961; Fig. 1). Even in this initial study, Mauro hypothesized that the satellite cell population may be involved in regeneration and growth of its surrounding tissue. Satellite cells were shown to function as myoblasts by Reznik (1969), who was the first to label them as stem cells. This role was supported by Moss & Leblond (1970, 1971), who demonstrated through radioactive thymidine incorporation experiments that satellite cells became mitotically active and contribute nuclei to both damaged and growing myofibres. In a landmark study, Snow (1977b) demonstrated that satellite cells and not myofibres contribute nuclei to regenerating skeletal muscle. Following the in vitro culture of individually dissected myofibres, satellite cells survived the dispersal process and were stimulated to divide, forming clones, whereas the fibres themselves degenerated, showing the satellite cell as a robust population able to survive myofibre degeneration and proliferate (Bischoff, 1975).
The mechanism of satellite cell activation remained unexplored until Bischoff (1986) showed satellite cells on single isolated myofibres were stimulated to divide in response to a mitogen originating from crushed skeletal muscle, suggesting external factors act upon the quiescent satellite cell. Further studies on satellite cell responses to known external factors ascertained that transforming growth factor beta (TGF-β) inhibited their differentiation through the myogenic lineage (Allen & Boxhorn, 1987). Moreover, fibroblast growth factor (FGF) was shown to increase satellite cell proliferation and perturb differentiation whereas insulin-like growth factor-1 (IGF-1) was seen to slightly increase proliferation and increase differentiation of the lineage (Allen & Boxhorn, 1989). Bischoff (1990) showed that satellite cells in contact with myofibres in culture have a reduced mitogenic response compared to isolated cells, suggesting the myofibre regulates the initial satellite cell activation response, providing the first evidence that the niche environment is crucial in controlling the behaviour of the satellite cell.
The nature of satellite cell research changed dramatically following the discovery of specific satellite cell molecular markers enabling their identification. Satellite cells have been shown to express the cell adhesion protein M-cadherin, along with other markers including the myogenic regulatory factor Myf5, the tyrosine receptor kinase C-Met, CD34 and, more recently, the caveolae-forming protein Caveolin-1, and the cell surface proteins Syndecan-3 and 4 (Irintchev et al. 1994; Allen et al. 1995; Cornelison & Wold, 1997; Beauchamp et al. 2000; Cornelison et al. 2001; Volonte et al. 2005). Most importantly, the paired box transcription factor Pax7 is expressed in the nucleus of all quiescent adult satellite cells and is necessary for their maintenance as a viable population during adulthood (Seale et al. 2000; Oustanina et al. 2004, see Table 1).
Pax genes and the satellite cell lineage
The ‘Paired Box’, or Pax, family of genes function as transcription factors and play essential roles during embryonic development (Bopp et al. 1986; Treisman et al. 1991; Mansouri et al. 1999). There are nine known Pax genes (Pax1-9) in mammals that are divided into four subgroups based on genomic structure, sequence similarity and function (Walther et al. 1991). Pax3 and Pax7 are homologous genes that make up one of the four subgroups. Despite their structural homology, Pax3 and Pax7 have differing functions during embryological development. In the mouse and chick, Pax3 and Pax7 are expressed throughout the embryonic dorsal neural tube and in the neural crest cells (Goulding et al. 1991; Mansouri et al. 1996; Lacosta et al. 2005). Mice homozygous for the Pax3 spontaneous mutant ‘splotch’ die during gestation and harbour numerous neural crest deficiencies, particularly in the caudal region of the embryo (Auerbach, 1954). Conversely, Pax7-null mice exhibit anterior (cephalic) neural crest defects and most die within 3 weeks of birth; however, some survive until adulthood (Mansouri et al. 1996; Oustanina et al. 2004).
One major role for Pax3 and Pax7 genes in the embryo is in the myogenic pathway, and particularly the specification of myogenic stem cells. In the embryo, Pax3 is expressed in the developing presomitic mesoderm; however, it becomes localized to the dermamyotome as the somite differentiates (Goulding et al. 1991), and is strongly expressed in the epaxial and hypaxial lips (Goulding et al. 1994). Pax3 mutant mice show somite formation defects and an absence of the hypaxial dermamyotome, leading to trunk muscle defects (Tremblay et al. 1998; Schubert et al. 2001). Moreover, Pax3 is essential for expression of the C-Met tyrosine kinase receptor in the hypaxial somite, necessary for myoblast migration (Bladt et al. 1995; Epstein et al. 1996), and subsequently Pax3-null mice show no migration of muscle precursors from the hypaxial dermamyotome into the limbs (Goulding et al. 1994). There is no rescue of hypaxial myogenic cell migration when Pax3 is replaced by Pax7 in the mouse, showing apparent specific roles for the two genes, and they may have diverged following the duplication of a common Pax3/7 precursor gene in response to the requirements of appendage muscle formation (Relaix et al. 2004). Tajbakhsh et al. (1997) demonstrated that Pax3 is involved in controlling the entry of precursor cells to the myogenic lineage through acting upstream of the myogenic regulatory factor MyoD in the mouse embryo. Further investigations analysing the Myf5 promoter sequence revealed that Pax3 binds to a 145-base pair element that is essential for Myf5 transcription in the hypaxial somite, the hypoglossal cord and the limbs (Bajard et al. 2006).
Pax7-null mice showed no skeletal muscle developmental defects, suggesting a redundancy through Pax3 which is sufficient to maintain myogenesis in the embryo (Mansouri et al. 1996). However, Pax7 is expressed in the developing dermamyotome, suggesting that a function for Pax7 exists during myogenesis (Jostes et al. 1990). Seale et al. (2000) failed to detect satellite cells in Pax7-null mice, suggesting an essential role for Pax7 in the specification of the satellite cell lineage in vertebrates. However, subsequent studies showed the presence of satellite cells (although infrequent) in Pax7-null animals, suggesting Pax7 is essential for the renewal and propagation of the satellite cell population throughout embryogenesis and adulthood (Oustanina et al. 2004). Pax7 has been shown to act as a cell survival signal for satellite cells through the prevention of the expression of the myogenic regulatory factors MyoD and myogenin (Olguin & Olwin, 2004; Relaix et al. 2006). Furthermore, myogenin has been shown to down-regulate Pax7 expression, suggesting that a reciprocal inhibition may exist between Pax7 and the myogenic regulatory factors, and that Pax7 must be down-regulated to allow myogenic differentiation to proceed (Olguin et al. 2007). However, Zammit et al. (2006) showed that Pax7 over-expression is permissive for MyoD and myogenin expression in satellite cell cultures, although myogenin onset was delayed. Moreover, it has been shown that Pax7 over-expression in the Pax7-null murine C2C12 myogenic cell line up-regulated MyoD expression but perturbed differentiation (Zammit et al. 2006). Together these data suggest a role for Pax7 in maintaining satellite cell proliferation but not inducing quiescence. The presence of satellite cells in Pax7-null animals would suggest that an amount of redundancy exists between Pax3 and Pax7 in the specification of satellite cells. Indeed, Pax3 is expressed in a subset of the adult satellite cell lineage, which includes cells from the diaphragm and 50% of forelimb muscles. Moreover, Pax3 has been used as a marker to isolate pure populations of adult satellite cells from the diaphragm of Pax3GFP/+ mice (Conboy & Rando, 2002; Buckingham et al. 2003; Montarras et al. 2005; Relaix et al. 2006). However, the majority of hind limb satellite cells are negative for Pax3 and hind limb musculature shows a down-regulation of Pax3 expression in certain muscles during fetal development (Otto et al. 2006; Relaix et al. 2006). Moreover, in Pax7-null mice, satellite cells are lost progressively during adulthood in Pax3-expressing and -non-expressing muscle through apoptosis showing that Pax3 cannot substitute for the anti-apoptotic role of Pax7 in vivo (Relaix et al. 2006). C57/BL6 Pax7-null mice die at 2–3 weeks of age, however, through back crossing onto the 129/Sv/J genetic background, Kuang et al. reported viable Pax7-null mice (Mansouri et al. 1996; Seale et al. 2000; Kuang et al. 2006). These mice showed extensive muscle wasting and severely perturbed regeneration, showing a necessity for Pax7 in muscle growth and repair. Pax3+ satellite cells were present in the Pax7-null mice but interestingly were not detected in the hind limbs. Intriguingly, they reported a Pax3+ side population of non-sub-laminar cells and suggested a distinct role for Pax3 in this separate population (see side population cells).
Embryonic origins of satellite cells
Through classical embryological experimentation using the chick as a model system it has been known for many years that the vertebrate skeletal muscle of the trunk and limbs originates in the somites (reviewed by Scaal & Christ, 2004). Only recently, however, has the origin of skeletal muscle satellite cells been distinguished. Using Pax3 and Pax7 as satellite cell markers, recent studies using green fluorescent protein (GFP) lineage tracing and chick-quail transplantation techniques have identified a population of myogenic precursor cells that originate in the central portion of the dermamyotome, persist in a proliferating, undifferentiated progenitor state as the dermamyotome breaks down and become interspersed within the differentiated myogenic cells of the myotome, where they continue to be a source for embryonic and fetal muscle (Gros et al. 2005). Moreover, it has been shown that these cells remain as progenitors throughout late fetal development, and subsequently become enveloped beneath the basal lamina of developing myofibres where they take up the position of quiescent satellite cells in late fetal and post-natal trunk muscle, demonstrating a somitic origin for trunk satellite cells in vertebrates (Gros et al. 2005; Kassar-Duchossoy et al. 2005; Relaix et al. 2005).
Traditional dogma states that embryonic and fetal muscle develops through two successive stages, primary and secondary myogenesis, and that these processes are carried out by primary and secondary myoblasts, respectively (Feldman & Stockdale, 1992). Prior to satellite cell lineage tracing experiments, it was thought that satellite cells emerged as a final population of myoblasts during late fetal development (Feldman & Stockdale, 1992). However, as discussed in the previous section, it has been demonstrated that satellite cells emerge from a population of undifferentiated stem cells that originate in the embryonic dermamyotome (Gros et al. 2005; Kassar-Duchossoy et al. 2005; Relaix et al. 2005). Moreover, the relationship between the satellite cell lineage and the primary and secondary myoblasts has been established, in that both primary and secondary myogenic regulatory factor-positive (MRF+) myoblast populations arise from the pool of Pax3+/Pax7+ myogenic precursor cells throughout development (Relaix et al. 2005) and neither primary or secondary myoblast populations go on to form satellite cells in the adult. However, distinctions between the pool of Pax3+/Pax7+ precursor cells that exist during embryonic and fetal development have been uncovered. Uncoupling of embryonic and fetal myogenesis using MyoD : Myf5 double mutant mice lines that only form embryonic but not fetal muscle and Mrf4nlacZ mutants to trace MRF4+ cells have shown that embryonic but not fetal precursor cells (from the Pax3+/Pax7+ pool) also express MRF4 and are therefore genetically distinct populations (Kassar-Duchossoy et al. 2005). Fetal Pax7+Myf5− and Pax7+/Myf5+ precursor cells have both been found in normal fetal muscle, suggesting that the two populations are in the same lineage, as observed in the adult (Kassar-Duchossoy et al. 2005; Kuang et al. 2007). Moreover, in Pax3sp/sp: Myf5nlacZ/nlacZ mutants that show no embryonic or fetal myogenesis (and therefore are devoid of embryonic and fetal myoblasts), fetal Pax7+/Myf5+ precursor cells are also observed and are therefore a distinct population of cells from the fetal myoblasts (Kassar-Duchossoy et al. 2005). These elegant genetic studies have clearly revealed the lineage progression of myogenic cells from Pax3+/Pax7+/MRF− progenitors to primary and secondary myoblasts.
Further studies have also traced the origins of limb muscle satellite cells back to the embryonic dermamyotome. We have previously shown using the chick model that Pax3+ and Pax7+ myogenic stem cells migrate from the hypaxial somite into the limb bud mesenchyme (Otto et al. 2006). Moreover, it has been demonstrated that Pax3+ migrating myoblasts undergo a similar migration process into the limb bud mesenchyme in the mouse, following which they up-regulate Pax7 expression and remain MRF-negative (Kassar-Duchossoy et al. 2005). Finally, retroviral labelling techniques and chick-quail transplant experiments in the avian model, along with somite-specific Pax3 cre mouse lines have convincingly demonstrated that all limb muscle satellite cells originate from Pax3+ cells of the hypaxial dermamyotome (Schienda et al. 2006).
The majority of muscles in the head arise from the cranial paraxial head mesoderm, and not from the somites (see Tzahor, 2009). It has been well documented that genetic differences are apparent between the programmes of skeletal muscle development in the head vs. the trunk and limbs of vertebrates. For example, mutant mice lines that show no development of trunk and limb muscles are still able to form embryonic and fetal muscles of the head (Rudnicki et al. 1993; Tajbakhsh et al. 1997), suggesting distinct lineages of myoblasts are responsible for both trunk and head muscle tissue formation. Head muscles also harbour satellite cells; however, until recently their embryonic origins were unknown. Harel et al. (2009) have elegantly demonstrated through retroviral labelling and chick-quail transplantation experiments that along with head musculature, the satellite cells from the head muscles also originate in the cranial paraxial mesoderm. Furthermore, through the use of Pax3Cre and MesP1Cre (MesP1, mesoderm posterior 1) mouse lines to mark trunk and head muscles, respectively, they demonstrate that the satellite cells from the trunk are derived from Pax3+ cells, whereas the satellite cells from the head muscles are negative in Pax3cre mice. Importantly, they show that satellite cells from non-migratory head muscles originate from MesP1+ cells and not from Pax3+ cells, demonstrating that head muscle satellite cells are genetically distinct in origin and lineage from those of the trunk and limb muscles (Harel et al. 2009). Furthermore, using Myf5Cre, Islet1Cre, Nkx2.5Cre, MesP1Cre and Pax3Cre mouse lines (Nkx2.5, NK2 transcription factor-related, locus 5) they demonstrate that separate muscles of the head show distinct lineages of myogenic cells: eye muscles (Myf5+, MesP1+); mastication muscles (Myf5+, MesP1+, Isl1+, Nkx2.5+) (Isl1, islet 1); and tongue muscles (Myf5+, MesP1+, Pax3+). Moreover, they then demonstrate that cells from the Isl1+ lineage form the satellite cells found only in the mastication muscles, not in the eye or tongue muscles. Additionally, using VE-CadCre mouse lines (marking cells of endothelial origin), they rule out the possibility that these Isl1+ satellite cells are endothelial in origin. Thus, these data show that satellite cells of the differing head muscles are developmentally linked to their embryonic muscle origins (Harel et al. 2009). Despite these differing genetic origins, when a single myofibre harbouring Isl1+ satellite cells from the head was transplanted into an injured tibialis anterior (TA) muscle of the limb, it readily formed nascent myofibres showing normal myogenic differentiation, suggesting the regeneration capacity of somite- and cranial paraxial mesoderm-derived satellite cells of differing genetic lineages is similar (Harel et al. 2009).
Adult satellite cells as the main source for skeletal muscle
Satellite cells are able to regenerate damaged skeletal muscle in vivo (Moss & Leblond, 1971; Snow, 1977a) and have been the targets for cell grafting therapy (reviewed by Morgan & Partridge, 2003). Attempts to graft FACS-isolated Pax3GFP/+ satellite cells directly into mdx TA muscle gave varied levels of cell incorporation (Montarras et al. 2005). For example, the expansion of the same FACS-isolated Pax3GFP/+ satellite cell pool in vitro prior to engraftment reduced the incorporation potential of the cells with fewer dystrophin+ myofibres detected (Montarras et al. 2005). Furthermore, in separate studies, a side population of stem cells isolated from skeletal muscle, bone marrow stem cells, and vasculature progenitors (mesoangioblasts and pericytes) has been shown to contribute to newly formed myofibres and enter the satellite cell niche with varying efficacies within regenerating skeletal muscle (Gussoni et al. 1999; Asakura et al. 2002; LaBarge & Blau, 2002; Polesskaya et al. 2003; Sampaolesi et al. 2006; Dellavalle et al. 2007; see following sections and Fig. 2). Following these studies, the role for the satellite cell as the true myogenic stem cell was questioned. However, it has subsequently been shown that satellite cells are the definitive muscle-regenerating cell population, as when just one single intact myofibre taken from 3F-nLacZ-2E mice [in which β-galactosidase (β-gal) reports expression of the myosin light chain 3F gene in the nuclei of fast myofibers] containing approximately seven satellite cells was engrafted into irradiated mdx dystrophic muscle (β-gal−/dystrophin−), thousands of β-gal+/dystrophin+ myonuclei on hundreds of myofibres within the engrafted muscle were generated, highlighting the huge regenerative potential of a minute population of satellite cells (Collins et al. 2005). Moreover, when the same procedure was carried out with a single Myf5nLacZ/+ myofibre in which Myf5+ satellite cells are also β-gal+, the population of satellite cells formed following the regenerative process was shown to have expanded 10-fold, demonstrating the cells harbour a great ability to self-renew. Furthermore, the engrafted stem cell population was stable and over time produced increased amounts of myofibres, albeit at muscle-specific varying rates, suggesting long-term engraftment potential of satellite cells in this manner unlike that seen for transplanted myoblasts (see satellite cells in skeletal muscle disease therapy). Critically, the progeny from one engrafted myofibre were able to re-enter the myogenic programme following further rounds of muscle injury, showing that the satellite cell pool not only efficiently replenishes damaged muscle but also repopulates its own stem cell pool, which is then capable of re-activation on further injury to carry out tissue repair (Collins et al. 2005). Finally, this study highlighted the fact that single, mechanically isolated satellite cells, separated from the myofibre, are equally able to engraft successfully into the host and form new myofibres following intramuscular injection as are satellite cells within their niche on the myofibre; just 150 single satellite cells incorporated into 10% of the newly formed muscles fibres. However, satellite cells isolated through enzymatic digestion were 1000-fold less efficient at forming new myofibres following injury (Sherwood et al. 2004a; Collins et al. 2005). This provides good evidence that the method of satellite cell isolation is crucial in the success of the engrafted cells to form new tissue. Collins et al. (2005) is the only study thus far to show a cell population, the satellite cells, is capable of not just forming new myofibres, or even new satellite cells, but is a self-renewing population that is able to continually propagate new skeletal muscle tissue. This study highlights the fact that if a cell exists within the satellite cell niche, it is not necessarily a prerequisite for further myogenic stem cell activity and that the satellite cell is approximately 1000-fold more efficient at forming skeletal muscle when delivered in an unchanged state from that which occurs in the normal niche environment.
Satellite cell lineage progression and self-renewal
As a tissue-specific stem cell population, the satellite cell must carry out two roles: it must provide differentiating progeny that will fuse with both new and damaged myofibres and also repopulate the stem cell niche through a self-renewal mechanism. These processes require one of two possible mechanisms to occur. Either a subpopulation of the satellite cell progeny must stop itself from progressing down the myogenic lineage and revert back to a quiescent state, or a subpopulation of the satellite cells must divide asymmetrically, thus forming both differentiating and stem cell progeny (Fig. 3). Both scenarios are not necessarily mutually exclusive and there is supporting evidence that both models may occur in vivo (Zammit et al. 2004; Kuang et al. 2007).
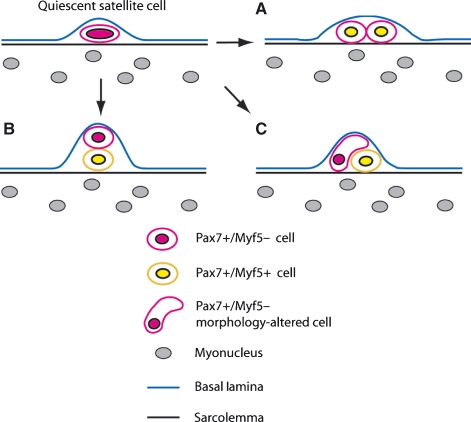
Fig. 3.
Diagram showing the proposed methods of satellite cell division. (A) Symmetric satellite cell division forming two equivalent daughter cells, both expressing Myf5. (B) Asymmetric division controlled by the niche environment with the true stem cell being juxtaposed against the basal lamina. (C) Asymmetric division brought about through morphological changes in the differentiating progeny, thus inducing these cells to inherit increased levels of certain differentiation-inducing proteins.
Following activation, the majority of satellite cells switch on the expression of the myogenic regulatory factor MyoD, and on differentiation they express myogenin (Koishi et al. 1995; Cornelison & Wold, 1997; Zammit et al. 2004). In a key study, Zammit et al. (2004) showed that a subset of activated satellite cells (i.e. MyoD+) switches off that marker while retaining Pax7 expression. These cells return to a quiescent state, suggesting that satellite cells replenish the niche environment through reversal of the myogenic lineage programme. However, recently satellite cells have also been shown to divide asymmetrically giving differential daughter cells (Conboy & Rando, 2002; Shinin et al. 2006; Kuang et al. 2007). Initial studies suggested that Notch-1 signalling was crucial in maintaining stem cell status within the satellite cell population with the antagonist to Notch, Numb, becoming segregated asymmetrically, inducing a heterogeneous population of progeny cells, with those expressing Numb undergoing MRF-induced differentiation (Conboy & Rando, 2002). Shinin et al. (2006) have suggested that satellite cells are capable of asymmetric division whereby one of the cells retains the maternal ‘immortal’ DNA strand – as proposed by Cairns (1975). The immortal strand hypothesis states that the true stem cell, when undergoing division, retains the template strand of DNA, while the newly synthesized strand, which would contain any mismatched base pairs, would be inherited by the cell destined for differentiation and therefore mitotic silence, thus reducing the likelihood for a build-up of potentially oncogenic mutations in the stem cell population (Cairns, 1975). However, it has been demonstrated that heterogeneity exists within the satellite cell population with only a subset of cells which can undergo asymmetric divisions and retain immortal DNA strands in this manner, suggesting not all Pax7+ quiescent satellite cells on adult myofibres have equivalent lineage status (Kuang et al. 2006; Shinin et al. 2006). Moreover, Beauchamp et al. (2000) showed that when identified through Myf5 expression, fewer satellite cells were seen on extensor digitorum longus (EDL) myofibres compared to the number of ‘negative’ satellite cells on myofibres marked with myosin light chain-specific 3F-nLacZ-2E transgene (marking all myonuclei of the fibre), suggesting that a majority, but not all, of the total satellite cell pool expresses Myf5. Using cre lox mouse lines expressing yellow fluorescent protein (YFP) under the Myf5 promoter, Kuang et al. (2007) were able to mark all cells that had once expressed Myf5 and found that approximately 10% of satellite cells had never expressed the gene (Kuang et al. 2007). Moreover, on initial division, this subset of cells underwent an asymmetric division, predominantly in the vertical plane to the myofibre, which resulted in one daughter cell becoming YFP+ and thus switching on Myf5, while the other daughter remained Myf5− (Kuang et al. 2007; see Fig. 3B). Importantly, cells dividing in the horizontal plane of the myofibre predominantly underwent symmetric divisions (Fig. 3A). Therefore it seems that the direction of cell separation and the alignment of the mitotic spindle may play a role in deciding whether the satellite cell forms two ‘differentiating’ daughter cells, or asymmetrically forming two different cell types. It was shown that the cell that remains in contact with the basal lamina of the myofibre will retain its stem cell status following asymmetric division, whereas the cell that loses contact initiates Myf5 expression and will subsequently differentiate (Fig. 3B). This highlights the role of the niche environment in controlling satellite cell proliferation, and is in keeping with the original idea from Bischoff (1990) showing that satellite cells kept in contact with the myofibre are less able to proliferate. The population of Myf5− satellite cells has been proposed as the true stem cells and are shown to repopulate the satellite cell niche at a far higher rate than Myf5+ cells when injected in vivo (Kuang et al. 2007).
However, asymmetric cell division can be achieved independently of nuclear position. Recently, it has been demonstrated that the ubiquitin ligase tripartite motif protein 32 (TRIM32) plays a key role in the asymmetric division within neuronal stem cells of the developing murine cortex through regulating the levels of cMyc within the cell population (Schwamborn et al. 2009). We have subsequently shown that TRIM32 is also necessary and sufficient to induce differentiation in the satellite cell lineage, where it acts through a similar pathway within the cell (J.C. Schwamborn, A. Otto, K. Patel and J. Knoblich, unpublished data). In cortical embryonic neuronal stem cells, asymmetric segregation of TRIM32 is not brought about through the alignment of the mitotic spindle, unlike the proposed model for satellite cells. Instead, the morphology of the cells differs as the cell divides, with one prospective daughter cell harbouring the morphology similar to a differentiating neuron and the other maintaining stem cell-like characteristics (Fig. 3C). Almost always, the TRIM32 protein segregates into the cell which resembles a differentiating neuron, regardless of cell alignment (Schwamborn et al. 2009). Therefore, we propose that a similar model could also be involved in the satellite cell lineage whereby altered morphology of one daughter cell within the niche environment causes the segregation of certain proteins such as TRIM32 to accumulate and subsequently causes only one of the daughter cells to undergo differentiation, perhaps through Myf5 initiation, leaving the second to remain as a true stem cell (Fig. 3C). In support of this model we have previously observed asymmetric segregation of TRIM32 within the satellite cell along with the protein Numb showing that, on asymmetric division, TRIM32 and Numb may segregate into the differentiating cell progeny (J. C. Schwamborn, A. Otto, K. Patel and J. Knoblich, unpublished data).
Regulation of satellite cell proliferation
Recently, we have demonstrated that canonical Wnt signalling, acting through the nuclear localization of β-catenin, plays a role in the early proliferative response of the satellite cell population to muscle damage (Otto et al. 2008). Recent work has also shown nuclear expression of β-catenin during this phase but has related the role of the Wnt pathway to the maintenance of the self-renewal programme within the satellite cells (Perez-Ruiz et al. 2008). Others have shown that the Wnt pathway also is involved in the differentiation of myoblasts, and that increased Wnt signalling following muscle damage leads to initial accelerated myofibre formation followed by an exhaustion of the myoblast pool and overall a perturbed regenerative response (Brack et al. 2008). We suggest that these seemingly incompatible results may be due to both the levels and timing of the Wnt signal during the muscle regenerative response. It is well established that canonical Wnts act to up-regulate the levels of the myogenic regulatory factor MyoD during embryonic skeletal muscle formation (Munsterberg et al. 1995; Stern et al. 1995; Wagner et al. 2000). Recently, we have shown that in the absence of MyoD there is a significant delay in the proliferative response of satellite cells immediately following myofibre isolation, along with a reduction in the levels of nuclear β-catenin (R. Macharia, A. Otto and K. Patel, unpublished data). Moreover, it has also been shown that the delay in regeneration due to the absence of MyoD is due to both perturbations in the levels of cell proliferation as well as differentiation (Megeney et al. 1996). There is strong molecular evidence suggesting that the levels of active MyoD protein within the satellite cell are crucial in regulating the rate of cell proliferation or differentiation, thus explaining the dual role for MyoD. Prior studies have shown that MyoD transcription within myoblasts in vitro and in vivo is positively regulated by serum response factor (SRF) that binds to the regulatory serum response element (SRE) upstream of the MyoD gene (Gauthier-Rouviere et al. 1996; L’Honore et al. 2003). More recently, L’Honore et al. reported that the SRE is a hybrid-binding element regulated through the binding both SRF (weakly) and myocyte enhancer factor 2 (MEF2) (strongly). During the myoblast proliferative phase, SRF regulates MyoD expression, causing low levels to build up in the cell and allowing proliferation to occur (L’Honore et al. 2007). Only prior to differentiation is MEF2 expression initiated and it out-competes SRF for the SRE binding site. MEF2 strongly induces MyoD expression, thus increasing the MyoD : Pax7 ratio and inducing differentiation (L’Honore et al. 2007). It has been shown that RhoA acts upstream of SRF to regulate MyoD and that the RhoA effector protein mDiaphanous either acts dependently on SRF or independently through β-catenin, positively regulating MyoD expression during C2C12 proliferation. Therefore β-catenin may act directly to regulate MyoD in the myoblast, as is observed in the embryo (Munsterberg et al. 1995; Carnac et al. 1998; Gopinath et al. 2007). Furthermore, it has also been demonstrated that active cyclin D1 (a Wnt β-catenin target gene) induces the nuclear expression of the cyclin-dependent kinase (Cdk) Cdk4. Cdk4 binds to MyoD directly, maintaining it in an inactive state, allowing cell cycle progression until the mitogenic signal is depleted. Following mitogen depletion, Cyclin D1 expression drops and the Cdk4 repression of MyoD activity is lost, allowing MyoD to function along with E-protein family members to induce myogenic gene transcription and myoblast differentiation (reviewed by Wei & Paterson, 2001).
Our data have shown that canonical Wnts are transcribed during the early satellite cell response to injury and that signalling through β-catenin in vitro can induce an early proliferative response in the satellite cell (Otto et al. 2008). However, Brack et al. (2008) have demonstrated that the levels of Wnt signalling in vivo slowly build up in skeletal muscle during the regenerative response to injury and are initially low. Therefore whether canonical Wnt proteins are expressed at sufficient levels directly following muscle injury to control satellite cell proliferation rates remains unclear. Much of the focus of recent research into satellite cell activation and proliferation has focused on the effects of nitric oxide (NO) in inducing hepatocyte growth factor (HGF) and insulin-like growth factor-1 (IGF-1) in mediating this response (Allen et al. 1995; Tatsumi et al. 1998; Sheehan et al. 2000). Both endogenous and neuronal nitric oxide synthase (NOS) proteins are expressed in skeletal muscle (Nakane et al. 1993; Kapur et al. 1997) and induce the activation of matrix metalloproteinases, causing the breakdown of the extra-cellular matrix (ECM) that contains bound HGF. Following the breakdown of the ECM, the HGF is released and acts upon its target receptor c-Met, robustly expressed on the satellite cell membrane (Tatsumi et al. 2006; Yamada et al. 2008, 2006; see Fig. 4C). It is known that the c-Met receptor can act through Akt, inhibit GSK-3 and stimulate the nuclear accumulation of β-catenin (Monga et al. 2002; Apte et al. 2006). Indeed, our experiments suggest that ectopic HGF augments the early accumulation of β-catenin within the satellite cell nucleus prior to initial division (A. Otto, and K. Patel, unpublished data). Therefore, in vivo, the early satellite cell proliferative response that involves nuclear β-catenin may be induced through HGF or IGF-1 signalling and not canonical Wnt (Fig. 4C).
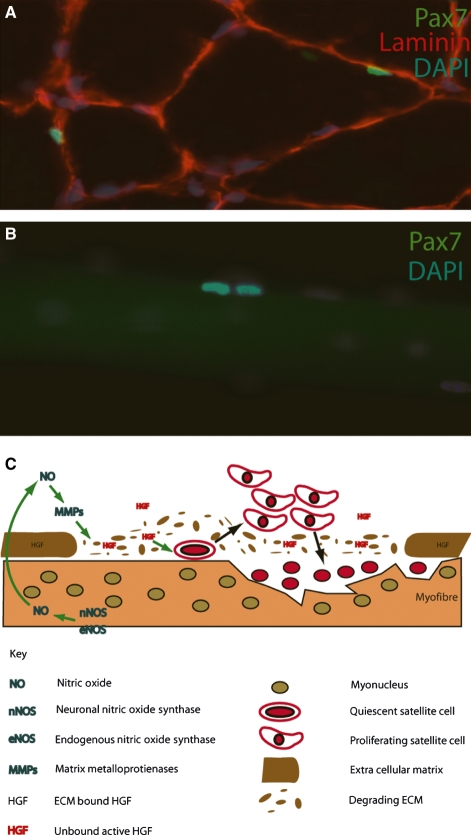
Fig. 4.
Satellite cell activation process is regulated through nitric oxide (NO)-mediated hepatocyte growth factor (HGF) release from the extra-cellular matrix (ECM). (A) Transverse cross-section through a mouse extensor digitorum longus muscle stained with antibodies against laminin (red) marking the basal lamina of individual myofibres. Quiescent Pax7+ (green) satellite cells are seen beneath the basal lamina. (B) Recently formed satellite cell doublet marked through Pax7 expression (green) on the surface of a mature adult myofibre. DAPI (blue) stains all the nuclei present in A and B. (C) Schematic showing how the regulation of satellite cell proliferation is regulated through NO production within the damaged myofibre, causing the activation of matrix metalloproteinases, the breakdown of the surrounding ECM and the subsequent release of bound HGF. HGF induces satellite cell progeny proliferation, thus regulating the regeneration response to injury.
Satellite cells in skeletal muscle disease therapy
The injection of pure populations of in vitro expanded, isolated primary murine myoblasts into damaged regenerating murine skeletal muscle has had mixed but never striking results, with little or no incorporation of injected cells into the satellite cell niche and a rapid loss (within 2–7 days) of the majority of injected myoblasts (Rando & Blau, 1994; Fan et al. 1996; Beauchamp et al. 1999). Further problems have arisen through the use of in vitro expanded myoblasts in disease therapy. Through systemic delivery, injected myoblast cells are unable to cross vasculature barriers and therefore require direct injection into the muscles of patients (Skuk et al. 2007). Although intra-muscular migration of myoblasts can be carried out easily, the area that the injected population can expand into is limited, and therefore multiple injections are required (Hughes & Blau, 1990; Skuk et al. 2007). Moreover, initial studies utilized immunosuppressive agents such as cyclosporin A, that were, first, inadequate and resulted in graft rejection, and secondly, acted to kill the injected cells as they underwent differentiation (Hardiman et al. 1993). The poor efficacy of myoblast cell grafts may be countered through the injection of high numbers of cells (up to 100 × 106) that indeed resulted in a reasonable level of dystrophin+ reconstituted myofibres in Duchenne muscular dystrophy (DMD) patients (Skuk et al. 1999, 2006). However, advancements in the efficacy of cell engraftment must be made to provide a sufficient level of symptom alleviation.
Mechanically isolated satellite cells show significant ability to repopulate the stem cell niche following intra-muscular injection (Collins et al. 2005; Montarras et al. 2005). It has been demonstrated that in the presence of the myofibre, satellite cells are less mitogenic when cultured in vitro, suggesting that the myofibre or satellite cell niche may be acting to perturb their division (Bischoff, 1990). Taken together with previously discussed works, these data suggest that contact with the niche environment is crucial in suppressing satellite cell division and that exposure to the external environment through the loss of their protective niche causes satellite cells to activate their intrinsically programmed myogenic cascade, thus losing their ability to produce new stem cell progeny able to return to a quiescent state beneath newly formed myofibres. Therefore the isolation of pure, non-activated populations of satellite cells would negate the need for large numbers of injected cells on treatment, reducing the invasiveness of the procedure (Montarras et al. 2005). Although isolated satellite cells show poor efficacy of engraftment following systemic delivery, inducing the need for intra-muscular injection, it has been suggested that the direct injection of cells into muscle would be a more favourable procedure with no risk of potential embolism occurring (Peault et al. 2007).
Bone marrow stem cells and muscle
The adult bone marrow contains a population of haematopoietic stem cells (HSC) that are known to form all the major constituent cell types of the blood system including erythrocytes, granulocytes and lymphocytes. Bone marrow also contains stromal cells [often called mesenchymal stem cells (MSC)] that are capable of forming osteoblasts, adipocytes, chondrocytes and fibroblasts (Prockop, 1997). Jiang et al. (2002) demonstrated that CD34−, CD44−, CD45−, c-Kit− multipotent adult progenitor cells (MAPCs) from bone marrow are able to differentiate into all three germ layers in vitro and form 45% chimaeric mice following blastocyst injection, indicating that bone marrow stem cells (BMSCs) harbour remarkable multipotent characteristics. Moreover, the skeletal muscle side population cells that are capable of haematopoietic reconstitution are derived from the bone marrow (Kawada & Ogawa, 2001a,b; McKinney-Freeman et al. 2002). Earlier cell culture studies revealed that BMSCs can differentiate into myotubes in vitro, showing they can potentially form skeletal muscle which was later shown to occur under normal myogenic culture conditions (Wakitani et al. 1995; Muguruma et al. 2003). Ferrari et al. (1998) showed for the first time that injection of whole bone marrow directly into injured skeletal muscle resulted in the incorporation of BMSCs into the newly forming skeletal muscle tissue. However, when compared to skeletal muscle satellite cells, BMSCs were less efficient at myotube fusion, suggesting they were not necessarily an appropriate cell type for potential therapeutic use. Crucially, however, unlike satellite cells, BMSCs maintained their stem cell characteristics when systemically delivered, and more readily pass through vascular walls into target tissues. Bone marrow contains a specific population of haematopoietic stem cells termed the side population, based on their ability to exclude Hoechst 33341 dye (Goodell et al. 1996). It was shown that following systemic injection of either whole BMSCs or the side population bone marrow fraction into irradiated mdx mice, dystrophin-expressing myofibres were observed within the muscle, showing that BMSCs were able to differentiate and fuse with existing myofibres through vascular delivery, and thus potentially are a key tool in treating hereditary muscular pathologies (Gussoni et al. 1999). However, Gussoni et al. (1999) reported that only the skeletal muscle-specific side population of stem cells was able to repopulate the satellite cell niche (a vital feature for any long lasting therapy – see side population section below), whereas whole BMSCs or bone marrow side population cells failed to do so. Despite BMSCs showing potential therapeutic value, the actual incorporation rates and number of myofibres expressing markers of injected cells remain extremely low and therefore below the level of patient acceptability in most studies reporting the use of these cells.
Following these key studies, many groups sought to elucidate the exact BMSC population able to repopulate damaged skeletal muscle and the mechanisms by which this process occurs. A number of differing BMSC fractions have been isolated and used in transplantation studies. A myogenic BMSC fraction expressing Pax3 and Myf5 has been reported, but showed no increase in dystrophin myofibre formation over that of whole bone marrow following injection into mdx mice (Corti et al. 2002). Using GFP+ BMSCs to lineage-trace transplanted cells, LaBarge & Blau (2002) showed convincingly for the first time that BMSCs are able to take up a position beneath the basal lamina of murine adult skeletal myofibres and express markers of satellite cells including Myf5, alpha7-integrin and c-Met. Interestingly, after injection of BMSCs, GFP+ myofibres were only observed following exercise-induced muscle damage. Importantly, BMSC-formed satellite cells were myogenic in culture, showed satellite cell characteristics and these cells were able to form GFP+ mature myofibres following injection into damaged skeletal muscle (LaBarge & Blau, 2002).
Although the level of cell engraftment into regenerating muscle tissue is very low, specific muscles incorporate systemically delivered BMSCs at largely varied rates with the panniculus carnosus (PC) muscle, showing the highest levels of BMSC-containing myofibres at 5% compared to the TA muscle (used for the majority of cardiotoxin studies) displaying on average just 0.07% (Brazelton et al. 2003). These results may reflect the differing rates of myofibre turn-over in specific muscles, as skeletal muscle stress or injury has been demonstrated to be a necessary prerequisite to BMSC engraftment (Abedi et al. 2004; Sherwood et al. 2004b; Palermo et al. 2005). Conflicting evidence exists as to whether BMSCs are capable of engrafting into normal healthy skeletal muscle without irradiation, chronic or acute injury responses occurring. However, one study has shown that BMSCs are able to engraft successfully into the TA muscle following exercise-induced muscle stress, suggesting that under normal circumstances this process may occur naturally (Palermo et al. 2005). Dreyfus et al. (2004) also showed that GFP+ BMSCs formed satellite cells and myofibres in uninjured but irradiated muscle, and these populations expanded with time following injection, suggesting that muscle injury is not a prerequisite for myofibre fusion of BMSCs. Efficiency or robustness of BMSC skeletal muscle incorporation has been assessed through various bone marrow (BM) transplantation methods. Irradiation and injury have been shown to increase cell incorporation into myofibre formation. Furthermore, mobilization of cells prior to injection through treatment with granulocyte colony-stimulating factor (G-CSF) enhanced myofibre formation, and direct injection into muscle gave engraftment levels of up to 12%, suggesting the limitation in myofibre engraftment could be through loss of cells following systemic delivery or inability of cells to cross the vasculature barrier (Abedi et al. 2004). However, there is convincing evidence that once engrafted into mature myofibres, BMSCs were reprogrammed to produce muscle protein; as human Nestin has been reported to be expressed in murine myofibres following transplantation of human BMSCs into mice (Lee et al. 2005). Nevertheless, these studies provide no evidence that the engrafted cell has become a bone marrow-derived satellite cell prior to myogenic differentiation, suggesting mononuclear cell fusion of the BMSC is then followed by intrinsic reprogramming.
To establish the exact bone marrow-derived progenitor cell type(s) that are able to undergo fusion into regenerating myofibres and whether these progenitors are also common for the haematopoietic lineage, two studies used a similar approach of injecting a single genetically marked progenitor cell along with an unmarked haematopoietic-depleted cell population into irradiated mdx mice. Single GFP+ c-Kit+ Lin1- Sca1+ or β-gal+CD45+Sca1+ haematopoietic BMSCs were shown to repopulate a significant proportion of the blood cell lineage as well as incorporate into newly forming skeletal muscle fibres (Camargo et al. 2003; Corbel et al. 2003). However, these studies provide conflicting evidence as to whether a regenerating skeletal muscle phenotype is necessary for myogenic fusion. Moreover, Camargo et al. (2003) suggest that no satellite cell niche incorporation occurs, and BMSCs fuse directly with the regenerating myofibre.
Further work has provided evidence that CD45+ C-Kit+Sca1+ Lin1-cell haematopoietic populations, and not mesenchymal stem cells, form myofibres following irradiation. However, it is unclear whether satellite cells are also established from transplanted cells (Abedi et al. 2004; Yoshimoto et al. 2005). Also, a study has highlighted a fraction of BMSCs that co-express CD45 and desmin present in human bone marrow and demonstrate that these cells can form myofibres in culture and following injection into injured TA muscle of nude mdx mice, albeit at very low frequencies (0.06–0.26% of TA fibres) (Bossolasco et al. 2004). However, in mice it has also been shown that CD45+ haematopoietic SP cells give rise to CD45−/desmin+ cells that are able to form muscle (Luth et al. 2008). Nevertheless, it is unclear whether the human CD45+/desmin+ population arises from a haematopoietic stem cell within the bone marrow or whether it exists as a separate population. FACS analysis of haematopoietic lineages showed that the Mac-1 (low) population of SP cells, high in myeloid progenitors, was able to reconstitute myofibres in vivo (Ojima et al. 2004). Moreover, it was further established more specifically that only c-Kit+ immature myelomonocytic progenitor cells that exclude Cd11b+ macrophages are capable of myofibre formation in vivo (Doyonnas et al. 2004).
There is evidence for bone marrow stromal cell myogenic conversion whereby C2C12 cells have been shown to fuse more readily to stromal lineages than to haematopoietic lineages in vitro and stromal cells are able to incorporate into myofibres in injured TA muscles from nude mice (Shi et al. 2004). Moreover, Dezawa et al. (2005) demonstrated that bone marrow stromal cells formed Pax7+ satellite cells that underwent numerous rounds of muscle regeneration. It is important to note that stromal cell populations exist in far higher numbers within the adult bone marrow and can be extracted in larger quantities than either haematopoietic c-Kit+ cells or indeed myogenic satellite cells from muscle tissue. Finally, adult human synovial membrane mesenchymal stem cells have been shown to incorporate into regenerating skeletal muscle from scid mice. Also, these cells can reconstitute dystrophin expression in mdx mice and take up satellite cell positions in muscle, suggesting they are also good candidate stem cells for use in potential human therapy (De Bari et al. 2003).
Recently, studies have shown that the signals inducing the conversion of BMSCs into skeletal muscle tissue arise specifically from injured muscle and are absent in uninjured tissue (Santa Maria et al. 2004). Certain groups have started to elucidate the specificity of these signalling cascades. Similarly to mesoangioblasts (see mesoangioblast section), chemokine receptor 4 (CXCR4), expressed on myoblasts, satellite cells and BMSCs, and its ligand stromal cell-derived factor-1 (SDF1), expressed on myofibroblasts within muscle tissue, could be involved in the in vivo guiding of BMSCs towards the site of muscle injury, as inhibition of CXCR4 signalling perturbs this process (Ratajczak et al. 2003). More recently, IGF-1 has been shown to increase the fusion of myelomonocytic BMSCs into skeletal muscle fourfold (Sacco et al. 2005). Moreover, Sun et al. (2009) provide evidence suggesting chemokine receptor 2 (CCR2) plays a role in recruitment of BMSCs into skeletal muscle, as CCR2−/− BMSCs display a reduced incorporation to damaged regenerating myofibres. However, a subpopulation of CD45+ BMSCs has been shown to migrate towards myocytes in response to HGF/c-Met signalling, but is refractory to SDF1/CXCR4 knockdown in this process, suggesting the subpopulation of cells is responsive to HGF in skeletal muscle and this population may be separate from that responsive to SDF1 (Rosu-Myles et al. 2005). Finally, in keeping with embryonic studies it has been demonstrated that Wnt proteins can induce a skeletal muscle phenotype from BMSCs. However, Wnts fail to form fully mature myofibres from such cells, suggesting other signals are also required for this process (Belema Bededa et al. 2005).
Despite a plethora of evidence showing that BMSCs have potential as therapeutic cells for treating genetic muscular disorders, several studies have shown the opposite. Lapidos et al. (2004)showed that following transplantation of wild type BMSCs into δ-sarcoglycan−/− mice, despite significant engraftment into host muscle tissue, insufficient levels of δ-sarcoglycan+ myofibres were formed to rescue the pathological phenotype. Moreover, when the haematopoietic fraction of BM was used to treat dystrophic dogs, no reconstitution of dystrophin expression was observed above that of normal myofibre reversion levels, even when cells were mobilized with G-CSF prior to injection (Dell’Agnola et al. 2004).
Skeletal muscle side population
The side population (SP) cells were initially isolated from skeletal muscle using the same methods that separated haematopoietic lineages from bone marrow through Hoechst 33342 dye exclusion (Goodell et al. 1996; Gussoni et al. 1999; Jackson et al. 1999). This side population of cells shows distinct characteristics that separate it from the bone marrow and satellite cell lineages. Studies have confirmed that skeletal muscle SP cells are negative for CD45 and C-Kit, markers of bone marrow SP cells, and positive for Stem Cell Antigen-1 (Sca-1), a marker not found on adult satellite cells, making them a distinct population that is not bone marrow-derived (Gussoni et al. 1999; Montanaro et al. 2004; Rivier et al. 2004). Moreover, Pax7−/− mice contain SP cells, but show greatly reduced levels of satellite cells (Seale et al. 2000). Kuang et al. (2006) demonstrated that viable Pax7−/− muscle showed extensive wasting and severely perturbed regeneration but maintained Pax3+ satellite cells in a subset of muscles, and also showed Pax3 expression within the SP, suggesting a distinct role for Pax3 in SP. However, other groups have not validated this finding. Furthermore, Pax7 can act to drive the myogenic capacity of SP cells to form skeletal muscle (Seale et al. 2004).
Recently, the origin of the majority of limb muscle SP cells has been traced to the somites and in particular the Pax3-expressing hypaxial somitic cells (Schienda et al. 2006). This study reported that only a very low percentage (0–5% depending on the sample and labelling method) of limb muscle SP cells express CD45, suggesting a small subset of these SP cells may have separate origins other than the somite, which could include endothelial cells, haematopoietic stem cells or bone marrow (Schienda et al. 2006). This already very low percentage is likely to be an over-estimate as the labelling techniques used in the study were not fully penetrant. Moreover, it was demonstrated that labelled somatically derived CD45-negative SP cells far more readily form myocytes when cultured in vitro than non-somitically derived SP cells, suggesting that the developmental origin of SP cells affects their intrinsic myogenic capacity, and the non-somitic subset of skeletal muscle SP cells has a lower myogenic potential (Schienda et al. 2006). These data all suggest that most, if not all, skeletal muscle SP cells are not of bone marrow origin (through CD45 expression) and the percentage of SP cells that originate from sources other than the somite show significantly lower myogenic capacity.
Skeletal muscle SP cells reside in the interstitial connective tissue of the skeletal muscle juxtaposed to blood vessels, express no myogenic markers and are non-myogenic in culture alone. However, when cultured with C2C12 myoblasts they initiate Pax7 expression, suggesting they have myogenic capacity (Gussoni et al. 1999; Asakura et al. 2002). Indeed, following direct injection into injured TA muscle, SP cells initiate expression of markers found in quiescent and activated satellite cells, including Pax7 and Myf5 (Asakura et al. 2002). The systemic administration of wild-type SP cells into mdx mice has revealed that SP cells have the ability to travel through small circulatory vessels within skeletal muscle and are able to repopulate the bone marrow of irradiated recipient animals, albeit to a lesser extent than haematopoietic bone marrow-derived SP cells (Gussoni et al. 1999). Importantly, skeletal muscle SP cells also incorporated into mdx regenerating myofibres, re-established dystrophin expression and showed satellite cell-like anatomical positioning beneath the basal lamina of the myofibres, indicating they can take up a position in the satellite cell niche (Gussoni et al. 1999; Asakura et al. 2002; Bachrach et al. 2004). Furthermore, 100 times fewer SP cells than main population cells are required to achieve similar levels of dystrophin expression in non-lethally irradiated mdx recipients, suggesting they have an increased capacity to incorporate into the stem cell compartment of the muscle (Muskiewicz et al. 2005). Although many studies have suggested that satellite cells and SP cells are two separate groups of progenitors, recent work has suggested that a degree of overlap occurs between the two. In an elegant study, Tanaka et al. (2009) have shown that SP cells, identified through Sca-1 and the ATP-binding cassette ABCG2, contain a subpopulation that also co-expresses syndecan-4, a marker for all satellite cells (Cornelison et al. 2001), and Pax7, suggesting they are akin to satellite cells; thus they have been termed satellite-SP cells. Moreover, satellite-SP cells are shown to reside beneath the basal lamina in the satellite cell position, and following direct injection into damaged TA muscle, they both incorporate into the myonuclear compartment of newly forming myofibres and re-populate the satellite cell niche (Tanaka et al. 2009). These data have parallels with the idea that the satellite cell population is heterogeneous in nature, with subsets of satellite cells showing lesser or greater stem cell-like status, with the latter harbouring a greater propensity to self-renew and repopulate the niche.
Muscle-derived stem cells (MDSC) – a separate muscle specific side population
Whereas SP cells are isolated and identified through FACS sorting on their ability to efflux the Hoechst 33342 dye, studies using a separate pre-plate technique to isolate and separate skeletal muscle stem cell types have revealed distinct populations of cells termed muscle-derived stem cells that exhibit differing characteristics and behaviours (Qu et al. 1998; Lee et al. 2000; Qu-Petersen et al. 2002). Initial studies identified separate populations of cells based on their temporal differences in binding to collagen-coated plates (Qu et al. 1998). Cells harvested at passage 1 showed low levels of desmin+ cells and were lost quickly following injection into regenerating muscle. However, by passage 6, cells contained high levels of desmin+ staining and showed a higher survival rate following muscle engraftment (Qu et al. 1998). Further characterization of these later passage cells showed that they are CD34+/Bcl2+ (B-cell lymphoma 2) and have the ability to undergo myogenic and osteogenic differentiation (Lee et al. 2000). Subsequently, it was established that a third population of progenitors could be obtained through extended pre-plating of the passage 6 cells (Torrente et al. 2001; Qu-Petersen et al. 2002). This cell population, termed MDSCs, displayed a greater propensity to form dystrophin+ myofibres than P1 cells following transplant into mdx muscle, bind to inter-muscular capillary networks following intra-arterial injection, and were able to self-renew in vitro and enter the satellite cell niche in vivo (Qu-Petersen et al. 2002).
Interestingly, it has been demonstrated that MDSCs can repopulate the haematopoietic lineage in irradiated murine hosts, and that these repopulated bone marrow cells can, first, repeat this process, and secondly, differentiate into myoblasts (Cao et al. 2003). Similar to studies involving SP cells, recent work has also shown that MDSCs can be isolated from Pax7−/− mice. However, without the restoration of Pax7 gene function, they fail to form myogenic progeny (Lu et al. 2008).
Mesoangioblasts
Through both electron microscopy and molecular cell-tracing methods, it has been well established that the satellite cell lineage in adult vertebrates originates from the dermomyotomal compartment of the somite (Armand et al. 1983; Gros et al. 2005; Relaix et al. 2005). However, other studies have identified myogenic potential in stem cells from other mesodermal tissues, in particular the dorsal aorta. These aortic cells express satellite cell markers and can be isolated from c-met−/− limb buds that contain no muscle tissue (Bladt et al. 1995; De Angelis et al. 1999). Termed mesoangioblasts, these cells have been shown to have an extensive developmental repertoire forming blood vessel wall, cartilage, smooth muscle, cardiac muscle and skeletal muscle following transplantation into chick embryos (De Angelis et al. 1999; Minasi et al. 2002). Mesoangioblasts express various stem cell markers including CD34, c-Kit and Flk1 (a marker of vasculature progenitors, see Table 1) but do not show expression of any lineage-specific or embryonic stem (ES) cell markers such as NKX2.5 (cardiac progenitors), Myf5 (skeletal muscle progenitors) or Oct4 (ES cells) and remain self-replicating over many passages of in vitro culture (Yamashita et al. 2000). Recently, however, it has been demonstrated that to form skeletal muscle, Pax3 expression within the mesoangioblast is required, showing that early skeletal muscle stem cell markers are initiated prior to differentiation and fusion into myofibres (Messina et al. 2008). Importantly, multiple-passaged clonal cell lines originating from mesoangioblasts maintain their potential to form several mesodermal tissues including bone, cartilage, smooth, cardiac and skeletal muscle following transplantation, demonstrating their true stem cell characteristics (Minasi et al. 2002).
Cre-lox experiments have established that Flk1-positive cells of the mouse naturally contribute to skeletal muscle development, suggesting that mesoangioblasts (that express Flk1) may be involved directly in skeletal muscle growth (Motoike et al. 2003). However, the contribution of these cells during normal adult growth is likely to be minimal. Nevertheless, several studies have shown that mesoangioblasts are capable of ameliorating the features of a number of differing skeletal muscle pathologies. First, Sampaolesi et al. (2003) showed that mesoangioblasts isolated from α-sarcoglycan−/− mice (a model for limb girdle muscular dystrophy) and lentiviral vector-transformed to re-express α-sarcoglycan protein were able to re-constitute normal skeletal myofibres in dystrophic animals following femoral artery injection. The efficiency of skeletal muscle reconstitution was unparalleled by any other cell-based therapy at the time and showed that mesoangioblasts have the essential abilities of donor cells to re-establish normal muscle pathology; both to travel unaffected throughout the circulatory system, and to cross the microvasculature cell wall where necessary and contribute robustly to repairing skeletal muscle tissue. Moreover, it has been shown that immuno-suppression was not necessary to allow this incorporation of allogeneic donor mesoangioblasts into host α-sarcoglycan-deficient dystrophic muscle, although immuno-suppressive drugs do increase the efficiency of this process (Guttinger et al. 2006).
Subsequently, mesoangioblast cells have been isolated from human patients with various muscular dystrophies including facioscapulohumeral muscular dystrophy (FSHD) and three separate inflammatory myopathies, one of which, inclusion-body myositis (IBM), harbours mesoangioblasts that fail to form skeletal muscle, and which is a disease that is disabling and has no current cure (Morosetti et al. 2006, 2007). Over-expression of MyoD within mesoangioblasts from human IBM patients, restores their ability to form skeletal muscle, therefore potentially presenting a cell therapeutic strategy for treating affected individuals without the need for immuno-suppression (Morosetti et al. 2006). In all other cases, mesoangioblasts have been isolated from diseased tissues and expanded in vitro and shown to undergo normal skeletal muscle differentiation (Morosetti et al. 2006, 2007).
Given the remarkable ability of mesoangioblast cells to reconstitute skeletal muscle following systemic cellular injection, a number of laboratories targeted potential signaling pathways that may further enhance their regenerative potential following treatment. To this end, Palumbo et al. (2004) showed that mesoangioblast migration from the circulatory vessel toward the injured wound/tissue sites is in part regulated by High Mobility Group Box-1 (HMGB1), a cytokine protein that is secreted by inflammatory cells within a wound site. Moreover, it has been demonstrated that another migratory-enhancing ligand/receptor pathway SDF-1/CXCR4 along with α-4 integrin enhance the migration of mesoangioblasts in a similar manner, and over-expression of these molecules prior to treatment significantly improves reconstitution of α-sarcoglycan−/− skeletal muscle with viable healthy myofibres (Galvez et al. 2006). Similar studies using the same murine dystrophic model have also shown that pretreatment of mesoangioblasts with nitric oxide significantly enhances α-sarcoglycan expression in a cyclic guanosine-mono-phosphate (GMP)-dependent manner and leads to a fivefold increase in treatment efficiency compared to control mesoangioblasts (Sciorati et al. 2006). Therefore, current technology is in place to utilize mesoangioblasts in effective cell therapeutics to alleviate dystrophic conditions.
Murine studies were then used as a paradigm to determine whether similar mesoangioblast cells from larger mammalian models of dystrophic pathologies could also be used to alleviate disease symptoms. Systemic delivery of canine mesoangioblasts significantly improved muscle morphology and physiological properties of diseased dogs through the restoration of dystrophin expression within skeletal muscle (Sampaolesi et al. 2006). As canine dystrophic muscle shows the same full spectrum of pathologies that are observed in human Duchenne muscular dystrophy patients, these data infer that the use of mesoangioblast cells in treating such disease states may be possible.
Pericytes
Pericytes (or Mural cells) reside in microvessels beneath the basement membrane adjacent to endothelial cells, where they are thought to aid the control of vessel size through smooth muscle actin contraction (Kutcher & Herman, 2009; see Fig. 2). Pericyte cells are of sclerotomal origin and can be separated from mesoangioblast cells on the basis of various molecular markers (Pouget et al. 2008). Adult human pericyte cells can be directly isolated through the expression of both N2 proteoglycan and alkaline phosphatase, neither of which is expressed by embryonic or adult mesoangioblasts or endothelial cells (Dellavalle et al. 2007). Previous studies have highlighted a large repertoire of cell types that pericytes can form following in vitro culture, including osteoblasts, chondrocytes, adipocytes and odontocytes, demonstrating them to be true multipotent stem cells (Schor et al. 1990; Doherty et al. 1998; Farrington-Rock et al. 2004; Alliot-Licht et al. 2005).
However, only two studies have used purified cultures of human pericyte cells and shown them to differentiate profusely into skeletal muscle tissue (Dellavalle et al. 2007; Crisan et al. 2008b). Importantly, it has been demonstrated that isolated human pericyte cells are distinct from satellite cells in that they do not express any early markers of the skeletal muscle stem cell lineage such as Pax7 or Myf5 and can be separated from satellite cells through FACS for alkaline phosphatase (ALP) and CD56 (neural cell adhesion molecule, N-CAM), with pericyte cells being ALP+/CD56–, or through CD146 and/or platelet-derived growth factor receptor (PDGFR)-β1 (Dellavalle et al. 2007; Schwab & Gargett, 2007; Crisan et al. 2008b). Also, human pericyte cells show no markers of haematopoietic lineages including CD34 and CD45 (Dellavalle et al. 2007; Crisan et al. 2008b). Moreover, human DMD pericytes were genetically very similar to control cells from healthy individuals and also differentiated into skeletal myotubes at a similar rate (Dellavalle et al. 2007). Interestingly, unlike satellite cells, pericyte precursors never expressed Pax7, Myf5 or MyoD during proliferative phases and only expressed lower levels of Myf5 and MyoD after differentiation had been induced (Dellavalle et al. 2007). Therefore in the pericyte lineage, separate mechanisms must be in place to regulate the levels of myogenin in the cell.
Pericyte cells have been isolated from various other human tissues including pancreas, adipose tissue and placenta. Nevertheless, all retain their myogenic potential when cultured in vitro (Crisan et al. 2008b). Expanding the importance of the pericyte cell was the discovery that in vitro cultured and in vivo adult human pericytes also express markers of mesenchymal stem cells including CD10, CD13, CD44, CD73 and CD90, and it has been proposed, based on these findings, that pericytes may be the source of mesenchymal stem cells in vivo (Crisan et al. 2008b).
Following interarterial delivery of human pericyte cells into scid-mdx mice, cells incorporated into regenerating myofibres, restored dystrophin expression and were able to incorporate into the satellite cell niche beneath the basal lamina of myofibres. Furthermore, pericyte cell therapy significantly improved the physiological performance of the skeletal muscle (Dellavalle et al. 2007). Moreover, mdx mutant pericyte cells can be easily manipulated in vitro to re-express non-mutated proteins prior to cell engraftment, where they contribute therapeutically to muscle tissue without the need for immune-suppressive therapy (Dellavalle et al. 2007).
Common origin for skeletal muscle and brown fat
Adipocytes share the same mesodermal origin with skeletal muscle (Rosen & MacDougald, 2006). An inverse relationship between skeletal muscle mass and adipose tissue mass is apparent in murine models of skeletal muscle dystrophic pathology such as mdx, where the relative level of fat tissue within the diseased muscle is increased (Anderson & Kunkel, 1992). Moreover, in the myostatin−/− mouse, where skeletal muscle mass is hugely increased, fat tissue mass is reduced substantially (McPherron et al. 1997). Moreover, cell culture studies have demonstrated that myogenic cell lines, when made to over-express adipogenic transcription factors ΠΠΑΡγ and C/ΕΒΠα lose their myogenic marker expression and differentiate into adipocytes, suggesting that adult myoblasts are capable being reprogrammed to become adipocytes (Hu et al. 1995). Furthermore, the satellite cell population has been shown to be capable of conversion into the adipocyte lineage given the correct cues (Asakura et al. 2001).
More recent work has provided good evidence that the adipocyte lineage can also undergo conversion into myoblasts. An adipocyte-specific stem cell population that expressed high levels of CD13, CD44, CD73 and CD90 and was negative for CD34, CD45, CD56 and CD184, suggesting mesenchymal stem cell-like characteristics, was recently isolated and shown to display myogenic markers and to fuse with maturing myofibres when co-cultured with myoblast cell lines (Lee & Kemp, 2006). Furthermore, it was demonstrated that cells isolated from the stromal vascular fraction (SVF) of adipose tissue, which have been shown to differentiate in vitro into adipogenic, chondrogenic, osteogenic and myogenic cells (Zuk et al. 2001), can spontaneously form myotubes when cultured under standard conditions in vitro (Di Rocco et al. 2006). Furthermore, these cells are able to fuse into myotubes following in vitro expansion and also, following injection into ischaemic murine hind limbs, form new myofibres and are capable of restoring dystrophin expression in mdx mice, thus displaying therapeutic potential (Di Rocco et al. 2006). More recently, a more specific CD45− side population of adipocyte progenitor cells purified from the SVF was shown to form myofibres in vivo (Andersen et al. 2008).
Array analysis of murine brown fat precursor cell populations has recently shown that certain myogenic transcription factors, including myogenin, Myf5 and MyoD, are expressed at levels comparable with C2C12 cells within these progenitors (Timmons et al. 2007). These data are in keeping with previous lineage-tracing studies that showed a dermomyotomal origin for brown, but not white, fat precursor cells (Atit et al. 2006). Moreover, brown fat lineage cells also express the known myogenic microRNAs miR-1 miR-133a and miR-206, suggesting that brown adipocytes share a common ancestor with myogenic cells (Walden et al. 2009).
Subsequently, it has been demonstrated that the transcription factor PRD1-BF1-RIZ1 homologous domain-containing 16 (PRDM16) is sufficient and necessary to drive the conversion of white adipocytes into brown adipocytes through up-regulation of uncoupling protein and peroxisome proliferator-activated receptor gamma (PPARγ) coactivator-1alpha (PGC1-α) expression, showing it to be a key regulator in the formation of the brown fat lineage (Seale et al. 2007). PRDM16 drives the brown fat lineage through forming a transcriptional complex with C-terminal binding protein 1 or 2, whereby it acts to repress white fat-specific genes, or complexing with PGC1-α, and PGC1-β whereby it enhances expression of brown fat genes (Kajimura et al. 2008). Recently, it has been elegantly shown that brown fat cells arise from an Myf5+ common precursor that was previously only thought to form skeletal muscle cells. Furthermore, PRDM16 over-expression causes brown fat cells to undergo a lineage switch, forming skeletal myoblasts through the activation of PPAR-γ, whereas PRDM16−/− brown fat has elevated myogenic gene transcription and reduced uncoupling ability (Seale et al. 2008). Subsequently, it has been shown that human skeletal muscle contains a population of brown fat precursor cells that up-regulates uncoupling protein 1 following PPAR-γ agonist treatment (Crisan et al. 2008a). Finally, human adipose tissue-derived mesenchymal stem cells have been shown to differentiate into myofibres spontaneously as well as induce dystrophin expression following co-culture with human DMD myoblasts in vitro through cell fusion (Vieira et al. 2008). These data show that adipogenic stem cells may be of use in therapeutic applications.
Conclusions
The use of stem cells in the clinical setting to treat skeletal muscle-wasting diseases as a therapy holds great potential. However, at present the efficacy of successful engraftment of stem cells, regardless of their origin or cell type, has mostly been shown to be below the level required to have significant beneficial impacts on disease states such as muscular dystrophies. In DMD patients, to alleviate symptoms of disease over long periods, the diseased stem cell population carrying mutations in the dystrophin protein must not only be replaced by a healthy set of cells, but these cells must engraft into the satellite cell niche and, crucially, be able to continue to form both healthy myofibres and self-renew. These two key factors would therefore abolish the need for any further treatment. Thus far, only Collins et al. (2005) have demonstrated that this can be achieved through the engraftment of satellite cells within their niche under the basal lamina of mature myofibres into injured skeletal muscle. First, satellite cells on a singular engrafted myofibre form multiple new myonuclei. Secondly, the engrafted stem cells repopulate the satellite cell niche, and thirdly, when transplanted further into damaged muscle, such labelled newly formed satellite cells are able to repeat the process in a separate recipient animal. This work provides substantial proof that the satellite cells possess all the necessary characteristics required for continued skeletal muscle regeneration, but it also provides an explanation for the fact that when satellite cells are isolated through enzymatic means, thus separating them from their niche environment and potentially damaging their cell surface receptors prior to direct intra-muscular or systemic engraftment, they are then unable to provide sufficient numbers of progenitor cells that harbour true stem cell status to ensure the treated muscle tissue maintains the ability to repair damage.
Given that satellite cells are highly sensitive when removed from the niche, and engraftment of mature satellite cell-containing myofibres into diseased skeletal muscle is unlikely to represent a feasible alternative, the systemic delivery of progenitor cells would seem the most logical avenue to pursue. This highlights the need to undertake investigations into the influence of the niche in controlling stem cell behaviour. Although Collins et al. (2005) provided evidence that satellite cells are the main source of regenerated muscle tissue when delivered under optimal conditions, this should not exclude the use of other cell types for therapeutic purposes. We suggest that alternative cell types such as HSC, mesoangioblasts, pericytes or adipocyte-derived stem cells, less sensitive to being isolated from their original niche than satellite cells, could be used for therapeutic applications. These cells may make up for their lower engraftment capacity by being able to be grown in large numbers while retaining both stem cell and myogenic potential, and importantly being able to cross the blood vessel walls, unlike satellite cells. Several cell types have been shown capable of forming both myofibres and engrafting into the satellite cell niche following systemic injection, including pericytes and mesoangioblasts, and therefore these cell types provide encouragement for the use of systemic stem cell injections with an end point of treating skeletal muscle disorders.
Acknowledgments
The authors would like to thank Dr W. R. Otto for critical reading of the manuscript. We are indebted to the University of Reading and Systems Biology Laboratory UK Ltd for generous funding.
References
- Abedi M, Greer DA, Colvin GA, et al. Robust conversion of marrow cells to skeletal muscle with formation of marrow-derived muscle cell colonies: a multifactorial process. Exp Hematol. 2004;32:426–434. doi: 10.1016/j.exphem.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Allen RE, Boxhorn LK. Inhibition of skeletal muscle satellite cell differentiation by transforming growth factor-beta. J Cell Physiol. 1987;133:567–572. doi: 10.1002/jcp.1041330319. [DOI] [PubMed] [Google Scholar]
- Allen RE, Boxhorn LK. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J Cell Physiol. 1989;138:311–315. doi: 10.1002/jcp.1041380213. [DOI] [PubMed] [Google Scholar]
- Allen RE, Sheehan SM, Taylor RG, et al. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J Cell Physiol. 1995;165:307–312. doi: 10.1002/jcp.1041650211. [DOI] [PubMed] [Google Scholar]
- Alliot-Licht B, Bluteau G, Magne D, et al. Dexamethasone stimulates differentiation of odontoblast-like cells in human dental pulp cultures. Cell Tissue Res. 2005;321:391–400. doi: 10.1007/s00441-005-1115-7. [DOI] [PubMed] [Google Scholar]
- Andersen DC, Schroder HD, Jensen CH. Non-cultured adipose-derived CD45- side population cells are enriched for progenitors that give rise to myofibres in vivo. Exp Cell Res. 2008;314:2951–2964. doi: 10.1016/j.yexcr.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Anderson MS, Kunkel LM. The molecular and biochemical basis of Duchenne muscular dystrophy. Trends Biochem Sci. 1992;17:289–292. doi: 10.1016/0968-0004(92)90437-e. [DOI] [PubMed] [Google Scholar]
- Apte U, Zeng G, Muller P, et al. Activation of Wnt/beta-catenin pathway during hepatocyte growth factor-induced hepatomegaly in mice. Hepatology. 2006;44:992–1002. doi: 10.1002/hep.21317. [DOI] [PubMed] [Google Scholar]
- Armand O, Boutineau AM, Mauger A, et al. Origin of satellite cells in avian skeletal muscles. Arch Anat Microsc Morphol Exp. 1983;72:163–181. [PubMed] [Google Scholar]
- Asakura A, Komaki M, Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation. 2001;68:245–253. doi: 10.1046/j.1432-0436.2001.680412.x. [DOI] [PubMed] [Google Scholar]
- Asakura A, Seale P, Girgis-Gabardo A, et al. Myogenic specification of side population cells in skeletal muscle. J Cell Biol. 2002;159:123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atit R, Sgaier SK, Mohamed OA, et al. Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol. 2006;296:164–176. doi: 10.1016/j.ydbio.2006.04.449. [DOI] [PubMed] [Google Scholar]
- Auerbach R. Analysis of the developmental effects of a lethal mutation in the house mouse. J Exp Zool. 1954;127:305–329. [Google Scholar]
- Bachrach E, Li S, Perez AL, et al. Systemic delivery of human microdystrophin to regenerating mouse dystrophic muscle by muscle progenitor cells. Proc Natl Acad Sci U S A. 2004;101:3581–3586. doi: 10.1073/pnas.0400373101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajard L, Relaix F, Lagha M, et al. A novel genetic hierarchy functions during hypaxial myogenesis: Pax3 directly activates Myf5 in muscle progenitor cells in the limb. Genes Dev. 2006;20:2450–2464. doi: 10.1101/gad.382806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp JR, Morgan JE, Pagel CN, et al. Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J Cell Biol. 1999;144:1113–1122. doi: 10.1083/jcb.144.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp JR, Heslop L, Yu DS, et al. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belema Bedada F, Technau A, Ebelt H, et al. Activation of myogenic differentiation pathways in adult bone marrow-derived stem cells. Mol Cell Biol. 2005;25:9509–9519. doi: 10.1128/MCB.25.21.9509-9519.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff R. Regeneration of single skeletal muscle fibers in vitro. Anat Rec. 1975;182:215–235. doi: 10.1002/ar.1091820207. [DOI] [PubMed] [Google Scholar]
- Bischoff R. A satellite cell mitogen from crushed adult muscle. Dev Biol. 1986;115:140–147. doi: 10.1016/0012-1606(86)90235-6. [DOI] [PubMed] [Google Scholar]
- Bischoff R. Interaction between satellite cells and skeletal muscle fibers. Development. 1990;109:943–952. doi: 10.1242/dev.109.4.943. [DOI] [PubMed] [Google Scholar]
- Bladt F, Riethmacher D, Isenmann S, et al. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- Bopp D, Burri M, Baumgartner S, et al. Conservation of a large protein domain in the segmentation gene paired and in functionally related genes of Drosophila. Cell. 1986;47:1033–1040. doi: 10.1016/0092-8674(86)90818-4. [DOI] [PubMed] [Google Scholar]
- Bossolasco P, Corti S, Strazzer S, et al. Skeletal muscle differentiation potential of human adult bone marrow cells. Exp Cell Res. 2004;295:66–78. doi: 10.1016/j.yexcr.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy IM, Conboy MJ, et al. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2008;2:50–59. doi: 10.1016/j.stem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Brazelton TR, Nystrom M, Blau HM. Significant differences among skeletal muscles in the incorporation of bone marrow-derived cells. Dev Biol. 2003;262:64–74. doi: 10.1016/s0012-1606(03)00357-9. [DOI] [PubMed] [Google Scholar]
- Buckingham M, Bajard L, Chang T, et al. The formation of skeletal muscle: from somite to limb. J Anat. 2003;202:59–68. doi: 10.1046/j.1469-7580.2003.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns J. Mutation selection and the natural history of cancer. Nature. 1975;255:197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- Camargo FD, Green R, Capetanaki Y, et al. Single hematopoietic stem cells generate skeletal muscle through myeloid intermediates. Nat Med. 2003;9:1520–1527. doi: 10.1038/nm963. [DOI] [PubMed] [Google Scholar]
- Cao B, Zheng B, Jankowski RJ, et al. Muscle stem cells differentiate into haematopoietic lineages but retain myogenic potential. Nat Cell Biol. 2003;5:640–646. doi: 10.1038/ncb1008. [DOI] [PubMed] [Google Scholar]
- Carnac G, Primig M, Kitzmann M, et al. RhoA GTPase and serum response factor control selectively the expression of MyoD without affecting Myf5 in mouse myoblasts. Mol Biol Cell. 1998;9:1891–1902. doi: 10.1091/mbc.9.7.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 2002;3:397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- Corbel SY, Lee A, Yi L, et al. Contribution of hematopoietic stem cells to skeletal muscle. Nat Med. 2003;9:1528–1532. doi: 10.1038/nm959. [DOI] [PubMed] [Google Scholar]
- Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol. 1997;191:270–283. doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- Cornelison DD, Filla MS, Stanley HM, et al. Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev Biol. 2001;239:79–94. doi: 10.1006/dbio.2001.0416. [DOI] [PubMed] [Google Scholar]
- Corti S, Strazzer S, Del BoR, et al. A subpopulation of murine bone marrow cells fully differentiates along the myogenic pathway and participates in muscle repair in the mdx dystrophic mouse. Exp Cell Res. 2002;277:74–85. doi: 10.1006/excr.2002.5543. [DOI] [PubMed] [Google Scholar]
- Crisan M, Casteilla L, Lehr L, et al. A reservoir of brown adipocyte progenitors in human skeletal muscle. Stem Cells. 2008a;26:2425–2433. doi: 10.1634/stemcells.2008-0325. [DOI] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008b;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- De Angelis L, Berghella L, Coletta M, et al. Skeletal myogenic progenitors originating from embryonic dorsal aorta coexpress endothelial and myogenic markers and contribute to postnatal muscle growth and regeneration. J Cell Biol. 1999;147:869–878. doi: 10.1083/jcb.147.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bari C, Dell’Accio F, Vandenabeele F, et al. Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane. J Cell Biol. 2003;160:909–918. doi: 10.1083/jcb.200212064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Agnola C, Wang Z, Storb R, et al. Hematopoietic stem cell transplantation does not restore dystrophin expression in Duchenne muscular dystrophy dogs. Blood. 2004;104:4311–4318. doi: 10.1182/blood-2004-06-2247. [DOI] [PubMed] [Google Scholar]
- Dellavalle A, Sampaolesi M, Tonlorenzi R, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- Dezawa M, Ishikawa H, Itokazu Y, et al. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science. 2005;309:314–317. doi: 10.1126/science.1110364. [DOI] [PubMed] [Google Scholar]
- Di Rocco G, Iachininoto MG, Tritarelli A, et al. Myogenic potential of adipose-tissue-derived cells. J Cell Sci. 2006;119:2945–2952. doi: 10.1242/jcs.03029. [DOI] [PubMed] [Google Scholar]
- Doherty MJ, Ashton BA, Walsh S, et al. Vascular pericytes express osteogenic potential in vitro and in vivo. J Bone Miner Res. 1998;13:828–838. doi: 10.1359/jbmr.1998.13.5.828. [DOI] [PubMed] [Google Scholar]
- Doyonnas R, LaBarge MA, Sacco A, et al. Hematopoietic contribution to skeletal muscle regeneration by myelomonocytic precursors. Proc Natl Acad Sci U S A. 2004;101:13507–13512. doi: 10.1073/pnas.0405361101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfus PA, Chretien F, Chazaud B, et al. Adult bone marrow-derived stem cells in muscle connective tissue and satellite cell niches. Am J Pathol. 2004;164:773–779. doi: 10.1016/S0002-9440(10)63165-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JA, Shapiro DN, Cheng J, et al. Pax3 modulates expression of the c-Met receptor during limb muscle development. Proc Natl Acad Sci U S A. 1996;93:4213–4218. doi: 10.1073/pnas.93.9.4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Maley M, Beilharz M, et al. Rapid death of injected myoblasts in myoblast transfer therapy. Muscle Nerve. 1996;19:853–860. doi: 10.1002/(SICI)1097-4598(199607)19:7<853::AID-MUS7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Farrington-Rock C, Crofts NJ, Doherty MJ, et al. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110:2226–2232. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Stockdale FE. Temporal appearance of satellite cells during myogenesis. Dev Biol. 1992;153:217–226. doi: 10.1016/0012-1606(92)90107-r. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Cusella-De Angelis G, Coletta M, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- Galvez BG, Sampaolesi M, Brunelli S, et al. Complete repair of dystrophic skeletal muscle by mesoangioblasts with enhanced migration ability. J Cell Biol. 2006;174:231–243. doi: 10.1083/jcb.200512085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier-Rouviere C, Vandromme M, Tuil D, et al. Expression and activity of serum response factor is required for expression of the muscle-determining factor MyoD in both dividing and differentiating mouse C2C12 myoblasts. Mol Biol Cell. 1996;7:719–729. doi: 10.1091/mbc.7.5.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, et al. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath SD, Narumiya S, Dhawan J. The RhoA effector mDiaphanous regulates MyoD expression and cell cycle progression via SRF-dependent and SRF-independent pathways. J Cell Sci. 2007;120:3086–3098. doi: 10.1242/jcs.006619. [DOI] [PubMed] [Google Scholar]
- Goulding MD, Chalepakis G, Deutsch U, et al. Pax-3, a novel murine DNA binding protein expressed during early neurogenesis. EMBO J. 1991;10:1135–1147. doi: 10.1002/j.1460-2075.1991.tb08054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding M, Lumsden A, Paquette AJ. Regulation of Pax-3 expression in the dermomyotome and its role in muscle development. Development. 1994;120:957–971. doi: 10.1242/dev.120.4.957. [DOI] [PubMed] [Google Scholar]
- Gros J, Manceau M, Thome V, et al. A common somitic origin for embryonic muscle progenitors and satellite cells. Nature. 2005;435:954–958. doi: 10.1038/nature03572. [DOI] [PubMed] [Google Scholar]
- Gussoni E, Soneoka Y, Strickland CD, et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- Guttinger M, Tafi E, Battaglia M, et al. Allogeneic mesoangioblasts give rise to alpha-sarcoglycan expressing fibers when transplanted into dystrophic mice. Exp Cell Res. 2006;312:3872–3879. doi: 10.1016/j.yexcr.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Hardiman O, Sklar RM, Brown RH., Jr Direct effects of cyclosporin A and cyclophosphamide on differentiation of normal human myoblasts in culture. Neurology. 1993;43:1432–1434. doi: 10.1212/wnl.43.7.1432. [DOI] [PubMed] [Google Scholar]
- Harel I, Nathan E, Tirosh-Finkel L, et al. Distinct origins and genetic programs of head muscle satellite cells. Dev Cell. 2009;16:822–832. doi: 10.1016/j.devcel.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu E, Tontonoz P, Spiegelman BM. Transdifferentiation of myoblasts by the adipogenic transcription factors PPAR gamma and C/EBP alpha. Proc Natl Acad Sci U S A. 1995;92:9856–9860. doi: 10.1073/pnas.92.21.9856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SM, Blau HM. Migration of myoblasts across basal lamina during skeletal muscle development. Nature. 1990;345:350–353. doi: 10.1038/345350a0. [DOI] [PubMed] [Google Scholar]
- Irintchev A, Zeschnigk M, Starzinski-Powitz A, et al. Expression pattern of M-cadherin in normal, denervated, and regenerating mouse muscles. Dev Dyn. 1994;199:326–337. doi: 10.1002/aja.1001990407. [DOI] [PubMed] [Google Scholar]
- Jackson KA, Mi T, Goodell MA. Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci U S A. 1999;96:14482–14486. doi: 10.1073/pnas.96.25.14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Jostes B, Walther C, Gruss P. The murine paired box gene, Pax7, is expressed specifically during the development of the nervous and muscular system. Mech Dev. 1990;33:27–37. doi: 10.1016/0925-4773(90)90132-6. [DOI] [PubMed] [Google Scholar]
- Kajimura S, Seale P, Tomaru T, et al. Regulation of the brown and white fat gene programs through a PRDM16/CtBP transcriptional complex. Genes Dev. 2008;22:1397–1409. doi: 10.1101/gad.1666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Bedard S, Marcotte B, et al. Expression of nitric oxide synthase in skeletal muscle: a novel role for nitric oxide as a modulator of insulin action. Diabetes. 1997;46:1691–1700. doi: 10.2337/diab.46.11.1691. [DOI] [PubMed] [Google Scholar]
- Kassar-Duchossoy L, Giacone E, Gayraud-Morel B, et al. Pax3/Pax7 mark a novel population of primitive myogenic cells during development. Genes Dev. 2005;19:1426–1431. doi: 10.1101/gad.345505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada H, Ogawa M. Bone marrow origin of hematopoietic progenitors and stem cells in murine muscle. Blood. 2001a;98:2008–2013. doi: 10.1182/blood.v98.7.2008. [DOI] [PubMed] [Google Scholar]
- Kawada H, Ogawa M. Hematopoietic progenitors and stem cells in murine muscle. Blood Cells Mol Dis. 2001b;27:605–609. doi: 10.1006/bcmd.2001.0426. [DOI] [PubMed] [Google Scholar]
- Koishi K, Zhang M, McLennan IS, et al. MyoD protein accumulates in satellite cells and is neurally regulated in regenerating myotubes and skeletal muscle fibers. Dev Dyn. 1995;202:244–254. doi: 10.1002/aja.1002020304. [DOI] [PubMed] [Google Scholar]
- Kuang S, Charge SB, Seale P, et al. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol. 2006;172:103–113. doi: 10.1083/jcb.200508001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S, Kuroda K, Le Grand F, et al. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutcher ME, Herman IM. The pericyte: cellular regulator of microvascular blood flow. Microvasc Res. 2009;77:235–246. doi: 10.1016/j.mvr.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Honore A, Lamb NJ, Vandromme M, et al. MyoD distal regulatory region contains an SRF binding CArG element required for MyoD expression in skeletal myoblasts and during muscle regeneration. Mol Biol Cell. 2003;14:2151–2162. doi: 10.1091/mbc.E02-07-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Honore A, Rana V, Arsic N, et al. Identification of a new hybrid serum response factor and myocyte enhancer factor 2-binding element in MyoD enhancer required for MyoD expression during myogenesis. Mol Biol Cell. 2007;18:1992–2001. doi: 10.1091/mbc.E06-09-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111:589–601. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- Lacosta AM, Muniesa P, Ruberte J, et al. Novel expression patterns of Pax3/Pax7 in early trunk neural crest and its melanocyte and non-melanocyte lineages in amniote embryos. Pigment Cell Res. 2005;18:243–251. doi: 10.1111/j.1600-0749.2005.00238.x. [DOI] [PubMed] [Google Scholar]
- Lapidos KA, Chen YE, Earley JU, et al. Transplanted hematopoietic stem cells demonstrate impaired sarcoglycan expression after engraftment into cardiac and skeletal muscle. J Clin Invest. 2004;114:1577–1585. doi: 10.1172/JCI23071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Kemp DM. Human adipose-derived stem cells display myogenic potential and perturbed function in hypoxic conditions. Biochem Biophys Res Commun. 2006;341:882–888. doi: 10.1016/j.bbrc.2006.01.038. [DOI] [PubMed] [Google Scholar]
- Lee JY, Qu-Petersen Z, Cao B, et al. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol. 2000;150:1085–1100. doi: 10.1083/jcb.150.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Kosinski PA, Kemp DM. Contribution of human bone marrow stem cells to individual skeletal myotubes followed by myogenic gene activation. Exp Cell Res. 2005;307:174–182. doi: 10.1016/j.yexcr.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Lu A, Cummins JH, Pollett JB, et al. Isolation of myogenic progenitor populations from Pax7-deficient skeletal muscle based on adhesion characteristics. Gene Ther. 2008;15:1116–1125. doi: 10.1038/gt.2008.86. [DOI] [PubMed] [Google Scholar]
- Luth ES, Jun SJ, Wessen MK, et al. Bone marrow side population cells are enriched for progenitors capable of myogenic differentiation. J Cell Sci. 2008;121:1426–1434. doi: 10.1242/jcs.021675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri A, Hallonet M, Gruss P. Pax genes and their roles in cell differentiation and development. Curr Opin Cell Biol. 1996;8:851–857. doi: 10.1016/s0955-0674(96)80087-1. [DOI] [PubMed] [Google Scholar]
- Mansouri A, Goudreau G, Gruss P. Pax genes and their role in organogenesis. Cancer Res. 1999;59:1707s–1709s. Discussion 1709s–1710s. [PubMed] [Google Scholar]
- Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney-Freeman SL, Jackson KA, Camargo FD, et al. Muscle-derived hematopoietic stem cells are hematopoietic in origin. Proc Natl Acad Sci U S A. 2002;99:1341–1346. doi: 10.1073/pnas.032438799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- Megeney LA, Kablar B, Garrett K, et al. MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev. 1996;10:1173–1183. doi: 10.1101/gad.10.10.1173. [DOI] [PubMed] [Google Scholar]
- Messina G, Sirabella D, Monteverde S, et al. Skeletal muscle differentiation of embryonic mesoangioblasts requires Pax3 activity. Stem Cells. 2008;27:157–164. doi: 10.1634/stemcells.2008-0503. [DOI] [PubMed] [Google Scholar]
- Minasi MG, Riminucci M, De Angelis L, et al. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development. 2002;129:2773–2783. doi: 10.1242/dev.129.11.2773. [DOI] [PubMed] [Google Scholar]
- Monga SP, Mars WM, Pediaditakis P, et al. Hepatocyte growth factor induces Wnt-independent nuclear translocation of beta-catenin after Met-beta-catenin dissociation in hepatocytes. Cancer Res. 2002;62:2064–2071. [PubMed] [Google Scholar]
- Montanaro F, Liadaki K, Schienda J, et al. Demystifying SP cell purification: viability, yield, and phenotype are defined by isolation parameters. Exp Cell Res. 2004;298:144–154. doi: 10.1016/j.yexcr.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Montarras D, Morgan J, Collins C, et al. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- Morgan JE, Partridge TA. Muscle satellite cells. Int J Biochem Cell Biol. 2003;35:1151–1156. doi: 10.1016/s1357-2725(03)00042-6. [DOI] [PubMed] [Google Scholar]
- Morosetti R, Mirabella M, Gliubizzi C, et al. MyoD expression restores defective myogenic differentiation of human mesoangioblasts from inclusion-body myositis muscle. Proc Natl Acad Sci U S A. 2006;103:16995–17000. doi: 10.1073/pnas.0603386103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morosetti R, Mirabella M, Gliubizzi C, et al. Isolation and characterization of mesoangioblasts from facioscapulohumeral muscular dystrophy muscle biopsies. Stem Cells. 2007;25:3173–3182. doi: 10.1634/stemcells.2007-0465. [DOI] [PubMed] [Google Scholar]
- Moss FP, Leblond CP. Nature of dividing nuclei in skeletal muscle of growing rats. J Cell Biol. 1970;44:459–462. doi: 10.1083/jcb.44.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss FP, Leblond CP. Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec. 1971;170:421–435. doi: 10.1002/ar.1091700405. [DOI] [PubMed] [Google Scholar]
- Motoike T, Markham DW, Rossant J, et al. Evidence for novel fate of Flk1+ progenitor: contribution to muscle lineage. Genesis. 2003;35:153–159. doi: 10.1002/gene.10175. [DOI] [PubMed] [Google Scholar]
- Muguruma Y, Reyes M, Nakamura Y, et al. In vivo and in vitro differentiation of myocytes from human bone marrow-derived multipotent progenitor cells. Exp Hematol. 2003;31:1323–1330. doi: 10.1016/j.exphem.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Munsterberg AE, Kitajewski J, Bumcrot DA, et al. Combinatorial signaling by Sonic hedgehog and Wnt family members induces myogenic bHLH gene expression in the somite. Genes Dev. 1995;9:2911–2922. doi: 10.1101/gad.9.23.2911. [DOI] [PubMed] [Google Scholar]
- Muskiewicz KR, Frank NY, Flint AF, et al. Myogenic potential of muscle side and main population cells after intravenous injection into sub-lethally irradiated mdx mice. J Histochem Cytochem. 2005;53:861–873. doi: 10.1369/jhc.4A6573.2005. [DOI] [PubMed] [Google Scholar]
- Nakane M, Schmidt HH, Pollock JS, et al. Cloned human brain nitric oxide synthase is highly expressed in skeletal muscle. FEBS Lett. 1993;316:175–180. doi: 10.1016/0014-5793(93)81210-q. [DOI] [PubMed] [Google Scholar]
- Ojima K, Uezumi A, Miyoshi H, et al. Mac-1(low) early myeloid cells in the bone marrow-derived SP fraction migrate into injured skeletal muscle and participate in muscle regeneration. Biochem Biophys Res Commun. 2004;321:1050–1061. doi: 10.1016/j.bbrc.2004.07.069. [DOI] [PubMed] [Google Scholar]
- Olguin HC, Olwin BB. Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: a potential mechanism for self-renewal. Dev Biol. 2004;275:375–388. doi: 10.1016/j.ydbio.2004.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olguin HC, Yang Z, Tapscott SJ, et al. Reciprocal inhibition between Pax7 and muscle regulatory factors modulates myogenic cell fate determination. J Cell Biol. 2007;177:769–779. doi: 10.1083/jcb.200608122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto A, Schmidt C, Patel K. Pax3 and Pax7 expression and regulation in the avian embryo. Anat Embryol (Berl) 2006;211:293–310. doi: 10.1007/s00429-006-0083-3. [DOI] [PubMed] [Google Scholar]
- Otto A, Schmidt C, Luke G, et al. Canonical Wnt signalling induces satellite-cell proliferation during adult skeletal muscle regeneration. J Cell Sci. 2008;121:2939–2950. doi: 10.1242/jcs.026534. [DOI] [PubMed] [Google Scholar]
- Oustanina S, Hause G, Braun T. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. EMBO J. 2004;23:3430–3439. doi: 10.1038/sj.emboj.7600346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo AT, Labarge MA, Doyonnas R, et al. Bone marrow contribution to skeletal muscle: a physiological response to stress. Dev Biol. 2005;279:336–344. doi: 10.1016/j.ydbio.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Palumbo R, Sampaolesi M, De Marchis F, et al. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol. 2004;164:441–449. doi: 10.1083/jcb.200304135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peault B, Rudnicki M, Torrente Y, et al. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol Ther. 2007;15:867–877. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- Perez-Ruiz A, Ono Y, Gnocchi VF, et al. Beta-catenin promotes self-renewal of skeletal-muscle satellite cells. J Cell Sci. 2008;121:1373–1382. doi: 10.1242/jcs.024885. [DOI] [PubMed] [Google Scholar]
- Polesskaya A, Seale P, Rudnicki MA. Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell. 2003;113:841–852. doi: 10.1016/s0092-8674(03)00437-9. [DOI] [PubMed] [Google Scholar]
- Pouget C, Pottin K, Jaffredo T. Sclerotomal origin of vascular smooth muscle cells and pericytes in the embryo. Dev Biol. 2008;315:437–447. doi: 10.1016/j.ydbio.2007.12.045. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Qu Z, Balkir L, van Deutekom JC, et al. Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol. 1998;142:1257–1267. doi: 10.1083/jcb.142.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu-Petersen Z, Deasy B, Jankowski R, et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851–864. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak MZ, Majka M, Kucia M, et al. Expression of functional CXCR4 by muscle satellite cells and secretion of SDF-1 by muscle-derived fibroblasts is associated with the presence of both muscle progenitors in bone marrow and hematopoietic stem/progenitor cells in muscles. Stem Cells. 2003;21:363–371. doi: 10.1634/stemcells.21-3-363. [DOI] [PubMed] [Google Scholar]
- Relaix F, Rocancourt D, Mansouri A, et al. Divergent functions of murine Pax3 and Pax7 in limb muscle development. Genes Dev. 2004;18:1088–1105. doi: 10.1101/gad.301004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Rocancourt D, Mansouri A, et al. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;435:948–953. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- Relaix F, Montarras D, Zaffran S, et al. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol. 2006;172:91–102. doi: 10.1083/jcb.200508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznik M. Thymidine-3H uptake by satellite cells of regenerating skeletal muscle. J Cell Biol. 1969;40:568–571. doi: 10.1083/jcb.40.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier F, Alkan O, Flint AF, et al. Role of bone marrow cell trafficking in replenishing skeletal muscle SP and MP cell populations. J Cell Sci. 2004;117:1979–1988. doi: 10.1242/jcs.01051. [DOI] [PubMed] [Google Scholar]
- Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- Rosu-Myles M, Stewart E, Trowbridge J, et al. A unique population of bone marrow cells migrates to skeletal muscle via hepatocyte growth factor/c-met axis. J Cell Sci. 2005;118:4343–4352. doi: 10.1242/jcs.02555. [DOI] [PubMed] [Google Scholar]
- Rudnicki MA, Schnegelsberg PN, Stead RH, et al. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell. 1993;75:1351–1359. doi: 10.1016/0092-8674(93)90621-v. [DOI] [PubMed] [Google Scholar]
- Sacco A, Doyonnas R, LaBarge MA, et al. IGF-I increases bone marrow contribution to adult skeletal muscle and enhances the fusion of myelomonocytic precursors. J Cell Biol. 2005;171:483–492. doi: 10.1083/jcb.200506123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaolesi M, Torrente Y, Innocenzi A, et al. Cell therapy of alpha-sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts. Science. 2003;301:487–492. doi: 10.1126/science.1082254. [DOI] [PubMed] [Google Scholar]
- Sampaolesi M, Blot S, D’Antona G, et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444:574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- Santa Maria L, Rojas CV, Minguell JJ. Signals from damaged but not undamaged skeletal muscle induce myogenic differentiation of rat bone-marrow-derived mesenchymal stem cells. Exp Cell Res. 2004;300:418–426. doi: 10.1016/j.yexcr.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Scaal M, Christ B. Formation and differentiation of the avian dermomyotome. Anat Embryol (Berl) 2004;208:411–424. doi: 10.1007/s00429-004-0417-y. [DOI] [PubMed] [Google Scholar]
- Schienda J, Engleka KA, Jun S, et al. Somitic origin of limb muscle satellite and side population cells. Proc Natl Acad Sci U S A. 2006;103:945–950. doi: 10.1073/pnas.0510164103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor AM, Allen TD, Canfield AE, et al. Pericytes derived from the retinal microvasculature undergo calcification in vitro. J Cell Sci. 1990;97(Pt 3):449–461. doi: 10.1242/jcs.97.3.449. [DOI] [PubMed] [Google Scholar]
- Schubert FR, Tremblay P, Mansouri A, et al. Early mesodermal phenotypes in splotch suggest a role for Pax3 in the formation of epithelial somites. Dev Dyn. 2001;222:506–521. doi: 10.1002/dvdy.1211. [DOI] [PubMed] [Google Scholar]
- Schwab KE, Gargett CE. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod. 2007;22:2903–2911. doi: 10.1093/humrep/dem265. [DOI] [PubMed] [Google Scholar]
- Schwamborn JC, Berezikov E, Knoblich JA. The TRIM-NHL protein TRIM32 activates microRNAs and prevents self-renewal in mouse neural progenitors. Cell. 2009;136:913–925. doi: 10.1016/j.cell.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciorati C, Galvez BG, Brunelli S, et al. Ex vivo treatment with nitric oxide increases mesoangioblast therapeutic efficacy in muscular dystrophy. J Cell Sci. 2006;119:5114–5123. doi: 10.1242/jcs.03300. [DOI] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, et al. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Seale P, Ishibashi J, Scime A, et al. Pax7 is necessary and sufficient for the myogenic specification of CD45+:Sca1+ stem cells from injured muscle. PLoS Biol. 2004;2:E130. doi: 10.1371/journal.pbio.0020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Kajimura S, Yang W, et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan SM, Tatsumi R, Temm-Grove CJ, et al. HGF is an autocrine growth factor for skeletal muscle satellite cells in vitro. Muscle Nerve. 2000;23:239–245. doi: 10.1002/(sici)1097-4598(200002)23:2<239::aid-mus15>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Sherwood RI, Christensen JL, Conboy IM, et al. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004a;119:543–554. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Sherwood RI, Christensen JL, Weissman IL, et al. Determinants of skeletal muscle contributions from circulating cells, bone marrow cells, and hematopoietic stem cells. Stem Cells. 2004b;22:1292–1304. doi: 10.1634/stemcells.2004-0090. [DOI] [PubMed] [Google Scholar]
- Shi D, Reinecke H, Murry CE, et al. Myogenic fusion of human bone marrow stromal cells, but not hematopoietic cells. Blood. 2004;104:290–294. doi: 10.1182/blood-2003-03-0688. [DOI] [PubMed] [Google Scholar]
- Shinin V, Gayraud-Morel B, Gomes D, et al. Asymmetric division and cosegregation of template DNA strands in adult muscle satellite cells. Nat Cell Biol. 2006;8:677–687. doi: 10.1038/ncb1425. [DOI] [PubMed] [Google Scholar]
- Skuk D, Roy B, Goulet M, et al. Successful myoblast transplantation in primates depends on appropriate cell delivery and induction of regeneration in the host muscle. Exp Neurol. 1999;155:22–30. doi: 10.1006/exnr.1998.6973. [DOI] [PubMed] [Google Scholar]
- Skuk D, Goulet M, Roy B, et al. Dystrophin expression in muscles of duchenne muscular dystrophy patients after high-density injections of normal myogenic cells. J Neuropathol Exp Neurol. 2006;65:371–386. doi: 10.1097/01.jnen.0000218443.45782.81. [DOI] [PubMed] [Google Scholar]
- Skuk D, Goulet M, Roy B, et al. First test of a ‘high-density injection’ protocol for myogenic cell transplantation throughout large volumes of muscles in a Duchenne muscular dystrophy patient: eighteen months follow-up. Neuromuscul Disord. 2007;17:38–46. doi: 10.1016/j.nmd.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Snow MH. Myogenic cell formation in regenerating rat skeletal muscle injured by mincing. I. A fine structural study. Anat Rec. 1977a;188:181–199. doi: 10.1002/ar.1091880205. [DOI] [PubMed] [Google Scholar]
- Snow MH. Myogenic cell formation in regenerating rat skeletal muscle injured by mincing. II. An autoradiographic study. Anat Rec. 1977b;188:201–217. doi: 10.1002/ar.1091880206. [DOI] [PubMed] [Google Scholar]
- Stern HM, Brown AM, Hauschka SD. Myogenesis in paraxial mesoderm: preferential induction by dorsal neural tube and by cells expressing Wnt-1. Development. 1995;121:3675–3686. doi: 10.1242/dev.121.11.3675. [DOI] [PubMed] [Google Scholar]
- Sun D, Martinez CO, Ochoa O, et al. Bone marrow-derived cell regulation of skeletal muscle regeneration. FASEB J. 2009;23:382–395. doi: 10.1096/fj.07-095901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajbakhsh S, Rocancourt D, Cossu G, et al. Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell. 1997;89:127–138. doi: 10.1016/s0092-8674(00)80189-0. [DOI] [PubMed] [Google Scholar]
- Tanaka KK, Hall JK, Troy AA, et al. Syndecan-4-expressing muscle progenitor cells in the SP engraft as satellite cells during muscle regeneration. Cell Stem Cell. 2009;4:217–225. doi: 10.1016/j.stem.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi R, Anderson JE, Nevoret CJ, et al. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol. 1998;194:114–128. doi: 10.1006/dbio.1997.8803. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Liu X, Pulido A, et al. Satellite cell activation in stretched skeletal muscle and the role of nitric oxide and hepatocyte growth factor. Am J Physiol Cell Physiol. 2006;290:C1487–C1494. doi: 10.1152/ajpcell.00513.2005. [DOI] [PubMed] [Google Scholar]
- Timmons JA, Wennmalm K, Larsson O, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci U S A. 2007;104:4401–4406. doi: 10.1073/pnas.0610615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrente Y, Tremblay JP, Pisati F, et al. Intraarterial injection of muscle-derived CD34(+)Sca-1(+) stem cells restores dystrophin in mdx mice. J Cell Biol. 2001;152:335–348. doi: 10.1083/jcb.152.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman J, Harris E, Desplan C. The paired box encodes a second DNA-binding domain in the paired homeo domain protein. Genes Dev. 1991;5:594–604. doi: 10.1101/gad.5.4.594. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Dietrich S, Mericskay M, et al. A crucial role for Pax3 in the development of the hypaxial musculature and the long-range migration of muscle precursors. Dev Biol. 1998;203:49–61. doi: 10.1006/dbio.1998.9041. [DOI] [PubMed] [Google Scholar]
- Tzahor E. Heart and craniofacial muscle development: a new developmental theme of distinct myogenic fields. Dev Biol. 2009;327:273–279. doi: 10.1016/j.ydbio.2008.12.035. [DOI] [PubMed] [Google Scholar]
- Vieira NM, Brandalise V, Zucconi E, et al. Human multipotent adipose-derived stem cells restore dystrophin expression of Duchenne skeletal-muscle cells in vitro. Biol Cell. 2008;100:231–241. doi: 10.1042/BC20070102. [DOI] [PubMed] [Google Scholar]
- Volonte D, Liu Y, Galbiati F. The modulation of caveolin-1 expression controls satellite cell activation during muscle repair. FASEB J. 2005;19:237–239. doi: 10.1096/fj.04-2215fje. [DOI] [PubMed] [Google Scholar]
- Wagner J, Schmidt C, Nikowits W, Jr, et al. Compartmentalization of the somite and myogenesis in chick embryos are influenced by wnt expression. Dev Biol. 2000;228:86–94. doi: 10.1006/dbio.2000.9921. [DOI] [PubMed] [Google Scholar]
- Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18:1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- Walden TB, Timmons JA, Keller P, et al. Distinct expression of muscle-specific microRNAs (myomirs) in brown adipocytes. J Cell Physiol. 2009;218:444–449. doi: 10.1002/jcp.21621. [DOI] [PubMed] [Google Scholar]
- Walther C, Guenet JL, Simon D, et al. Pax: a murine multigene family of paired box-containing genes. Genomics. 1991;11:424–434. doi: 10.1016/0888-7543(91)90151-4. [DOI] [PubMed] [Google Scholar]
- Wei Q, Paterson BM. Regulation of MyoD function in the dividing myoblast. FEBS Lett. 2001;490:171–178. doi: 10.1016/s0014-5793(01)02120-2. [DOI] [PubMed] [Google Scholar]
- Yamada M, Tatsumi R, Kikuiri T, et al. Matrix metalloproteinases are involved in mechanical stretch-induced activation of skeletal muscle satellite cells. Muscle Nerve. 2006;34:313–319. doi: 10.1002/mus.20601. [DOI] [PubMed] [Google Scholar]
- Yamada M, Sankoda Y, Tatsumi R, et al. Matrix metalloproteinase-2 mediates stretch-induced activation of skeletal muscle satellite cells in a nitric oxide-dependent manner. Int J Biochem Cell Biol. 2008;40:2183–2191. doi: 10.1016/j.biocel.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Yamashita J, Itoh H, Hirashima M, et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- Yoshimoto M, Chang H, Shiota M, et al. Two different roles of purified CD45+ c-Kit+Sca-1+ Lin-cells after transplantation in muscles. Stem Cells. 2005;23:610–618. doi: 10.1634/stemcells.2004-0220. [DOI] [PubMed] [Google Scholar]
- Zammit PS, Golding JP, Nagata Y, et al. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit PS, Relaix F, Nagata Y, et al. Pax7 and myogenic progression in skeletal muscle satellite cells. J Cell Sci. 2006;119:1824–1832. doi: 10.1242/jcs.02908. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]