Summary
The role of endomyocardial biopsies in patients with clinically suspected acute myocarditis, myocarditis in the past, and dilated cardiomyopathy is discussed controversially. In fact, it is still under discussion whether information obtained from endomyocardial biopsies is relevant for further clinical decisions. Therefore this Critical Perspective will deal with the question, which patient should undergo endomyocardial biopsy investigations for an etiopathogenic differentiation of the disease and for the possible choice of immunomodulatory treatment strategies.
Key words: Endomyocardial biopsy, myocarditis, dilated cardiomyopathy, inflammatory cardiomyopathy, viral infection
Introduction
The indication for endomyocardial biopsies (EMBs) in patients with clinically suspected acute myocarditis, myocarditis in the past, or dilated cardiomyopathy (DCM) is still being discussed controversially. One major reason is that no evidence-based guidelines exist to perform endomyocardial biopsies in these patients.
It is essential to differentiate between the acute (clinically suspected acute myocarditis) and the chronic phase of the disease (clinically suspected myocarditis in the past or DCM). In addition, it is important to emphasize that a differentiation between clinically based diagnosis and endomyocardial biopsy (EMB) proven diagnosis has to be taken into account, because there are no clinical symptoms — especially in the chronic phase of the disease — that correlate with the EMB results.
A detailed analysis of EMBs using contemporary diagnostic tools (exact characterization of the myocardial inflammation (histology according to Dallas classification, immunohistology) and of the viral persistence (molecular biological approach using polymerase chain reaction/PCR) is pertinent for the exact etiopathogenic differentiation [3, 9, 29, 31–33, 46–48, 50, 52, 56]. Histological assessment according to the Dallas classification enables the differentiated diagnosis of myocarditis (cellular infiltration combined with myocyte necrosis with or without fibrosis; Fig. 1) and of borderline myocarditis (cellular infiltration without myocyte necrosis with or without fibrosis). Once the diagnosis of myocarditis has been made in endomyocardial specimens, patients may be followed by repeat biopsy, especially if no improvement in left ventricular function is observed. In addition, if an unequivocal diagnosis of myocarditis has been made in a previous EMB, such terms as ongoing (persistent), resolving (healing), or resolved (healed) myocarditis with or without fibrosis in conjunction with the clinical course of the patient can be used to indicate progression or regression of disease [3]. In patients with global (left ventricular ejection fraction/LVEF <50%) or moderately impaired persistent LV dysfunction with locoregional wall motion abnormalities (LVEF >50%) and without histologically diagnosed myocarditis respectively borderline myocarditis according to the Dallas classification, further work-up concerning low grade myocardial inflammation and viral persistence is indicated. A more sensitive work-up for myocardial inflammation should be undertaken using highly sensitive immunohistological techniques for the detection of the low grade inflammation often seen in the chronic phase of disease consistent with inflammatory cardiomyopathy (DCMi) [56]. This low grade inflammation is, however, difficult to establish by conventional histology according to the Dallas criteria because the cellular infiltrates are often sparse and may be missed by sampling error. It is also difficult to distinguish between non-inflammatory cells (e.g., fibroblasts or pericytes) and infiltrating lymphocytes. By using immunohistological techniques, a more accurate identification of cellular infiltrates and other immune markers expressed in an active immunological process like cell adhesion molecules (e.g., intercellular cell adhesion molecule-1/ICAM-1, vascular cell adhesion molecule-1/VCAM-1) is feasible [5, 31, 48]. In addition, using higly sensitive molecularbiological tools, viral persistence can be diagnosed in these patients in high frequency. These different diagnostic tools, enable a classification of patients with inflammatory cardiomyopathy (DCMi: with immunohistologically proven myocardial inflammation in association with cardiac dysfunction according to the WHO Classification of Cardiomyopathies 1995) [39, 56] with and without viral persistence, in patients with viral persistence without myocardial inflammation, and in patients with idiopathic DCM based on genetic factors, healed myocarditis in the past, or storage diseases (e.g., amyloidosis; Fig. 2) [56] has to be considered on a case-by-case basis.
Fig. 1.
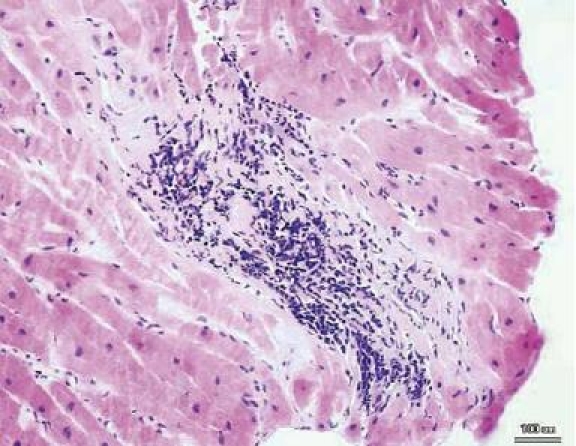
Myocarditis according to the Dallas classification. Focal lymphomono-nuclear infiltrates with adjacent myocytolysis (myocarditis; original magnification ×200). Reproduced from [53]
Fig. 2.
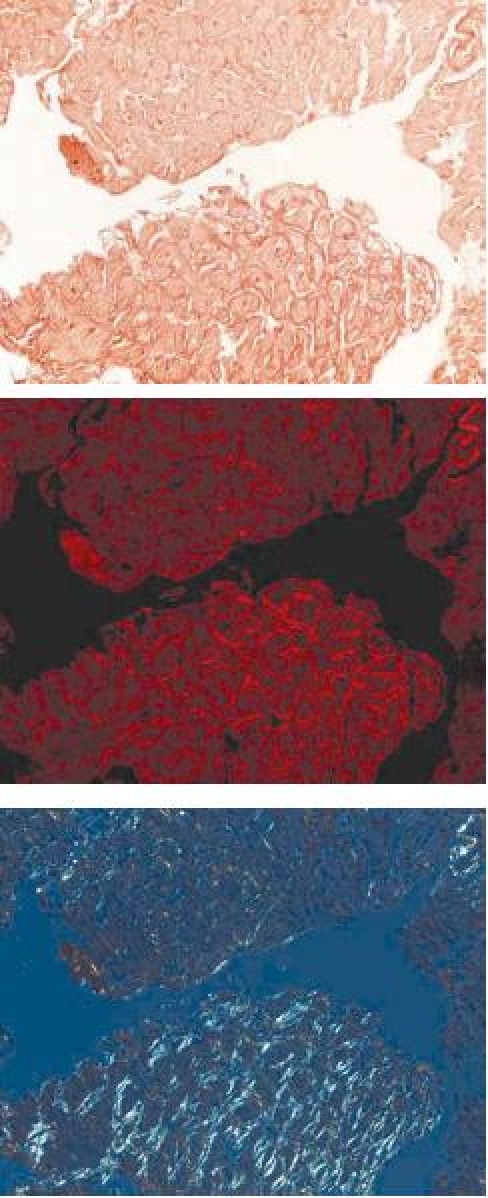
Cardiac amyloidosis: a Cardiac amyloidosis (H&E staining), b in polarized light, the Kongo red staining depicts amyloid fibrils at the surface and between cardiac myocytes, c same staining as b. In depolarized light, the amyloid fibrils can be identified as bright tissue areas
In patients with clinically suspected acute viral induced myocarditis, there is no definitive clinical indication to perform EMBs, because there are no data that an early specific treatment has any benefit concerning duration of the disease or improvement of heart failure. In addition, this disease has a quite good prognosis, because in about 80% of these patients a complete healing is observed [13]. However, in cases with progressive LV dysfunction of unknown cause despite complete heart failure medication, there is a need to perform EMB to verify the clinically suspected diagnosis of acute viral myocarditis and to rule out rare causes (e.g., giant cell myocarditis, eosinophilic myocarditis) for rapid progression of LV dysfunction with possible fatal course [1, 10–12, 15–17, 19, 23, 30, 49, 65, 68].
In contrast to the very early stage of acute myocarditis, the majority of EMB-based treatment trials were performed in patients with a more chronic course of disease like clinically suspected DCM. Therefore, in this stage of disease patients may benefit from a EMB-based diagnosis with respect to prognosis [35] and possible choice of rational treatment strategies (immunosuppression, antiviral treatment) (Fig. 4) based on monocentric or pilot trials [34, 36, 60, 69]. The approach of immunosuppression in patients with EMB proven myocardial inflammation without viral persistence has demonstrated beneficial effects of heart failure symptoms and LV function parameters [36, 69, 70], while adverse outcome of immunosuppression in virus positive patients has been reported [20]. The attempt of an antiviral treatment strategy is based on the known adverse prognostic impact of viral persistence with respect to the natural process of LV function and mortality [35]. An antiviral pilot trial with interferon-β demonstrated complete elimination of viral genomes in patients with adenoviral or enteroviral persistence, accompanied by a significant amelioration of clinical symptoms and significant improvement of LV function [34]. Based on these encouraging results, a prospective, placebo-controlled, multicenter, randomized phase II study (Betaferon® in Chronic Viral Cardiomyopathy — BICC Trial) is currently evaluating the clinical benefit of an interferon-β therapy in patients with viral persistence.
Fig. 4.
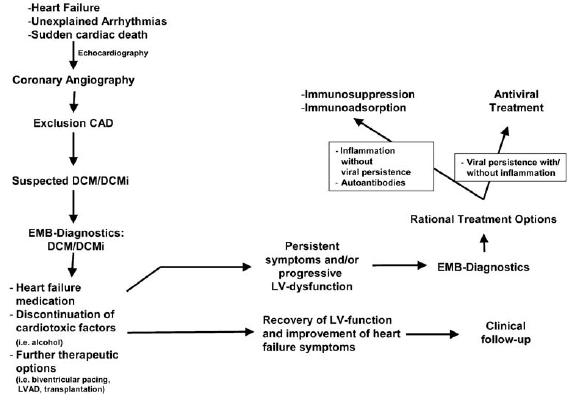
Diagnostic and therapeutic algorithms in DCM
Therefore, only a detailed analysis of EMBs concerning myocardial inflammation and viral persistence, respectively, allows the etiopathogenic differentiation and an implementation of causal treatment strategies in the context of clinical trials. This EMB-based approach may lead to improved patient management in the future.
This Critical Perspective will therefore deal with the question, which patients with clinically suspected acute myocarditis, clinically suspected myocarditis in the past, and DCM of unknown cause should undergo EMB investigations for an etiopathogenic differentiation of the disease and for the possible choice of immunomodulatory treatment strategies.
Background
Etiology of acute myocarditis
Acute myocarditis is most often caused by cardiotropic viral infections. As such, enteroviruses, particularly coxsackieviruses and adenoviruses, are known [9, 32, 49, 52]. An important step concerning the understanding of the molecular basis of cardiotropic viral infection was achieved by the studies analyzing the relevance of the coxsackievirus-adenovirus receptor (CAR) in this disease entity [45, 55]. In addition to enteroviruses and adenoviruses, recent investigations have shown that genomes of parvovirus B19, human herpes virus type 6, hepatitis C and Epstein-Barr virus are detected even more frequently in the myocardium of patients presenting with acute myocarditis [29, 33]. Interestingly, all these various cardiotropic viruses can be detected in patients with the clinically suspected diagnosis of DCM as well [20, 32]. With respect to enteroviruses, several pathomechanisms can contribute to the impairment and progression of LV function. These can be classified as direct virus-mediated and indirect virus-induced mechanisms. One recently elucidated direct virus-mediated effect is the cleavage of dystrophin by the enterovirus protease A2, thereby interfering with an essential part of the cardiomyocyte cytoskeleton responsible for force transmission [4]. Furthermore, even non-replicative intermediates of entero-viruses can cause an impairment of LV function in animal models [66]. Major virus-induced effects are exerted during the immune response to infection, which aims at viral elimination. Both lymphocytes and macrophages, the infiltration of which is mediated by the enhanced expression of cell adhesion molecules [46, 48], are involved in the course of the intramyocardial antiviral immune response. This cellular myocardial infiltration is linked to an increased expression of cytokines, which are known to impair LV function, e.g., tumor necrosis factor-α (TNF-α) [41, 63]. These pathogenic mechanisms involved in myocarditis and DCMi are summarized in Fig. 3.
Fig. 3.
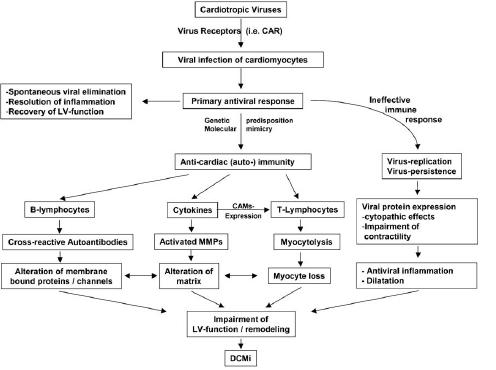
Pathophysiology of myocarditis and DCMi
Apart from viruses, bacteria, rickettsia, spirochaeta and fungi can be detected in special cases in patients presenting with clinically suspected acute myocarditis [23, 25]. A typical non-viral induced infection of the heart is caused by borrelia burgdorferi, which may preferentially interfere with the conduction system leading to conduction abnormalities [62].
Non-infective causes of myocardial inflammation and subsequent myocyte necrosis may be caused by cardiotoxic agents such as doxorubicin, systemic diseases with cardiac involvement (Lupus erythematosus [68] or other collagenoses) and occasionally as a consequence of a hypersensitivity reaction due to different drugs [23]. The drug-induced hypersensitivity reaction often presents as eosinophilic pancarditis with infiltrating lymphocytes, pathognomonic eosinophilic cells, plasma cells and histiocytes.
Giant cell myocarditis is another specific type of acute myocarditis of unknown origin. Due to the fatal prognosis, it is important to reach a defined histological diagnosis for further specific treatment approaches. The specific treatment approach in this disease entity is mainly based on a registry of 63 cases of giant cell myocarditis. The 22 patients treated with corticosteroids and cyclosporines, azathioprine, or both therapies survived for an average of 12.3 months, as compared with an average of 3 months for the 30 patients who received no immunosuppressive therapy (p<0.001). However, even these promising data concerning immunosuppressive therapy in these patients, heart transplantation is the final treatment of choice for most patients so far [10, 11]. In addition, it is very important to differentiate giant cell myocarditis from other forms of granulomatous diseases like cardiac sarcoidosis, because otherwise no adequate specific treatment approach can be initiated [10, 11, 49].
In rare cases, a hypersensitivity reaction caused by different drugs [23] can lead to the clinical picture of a hypersensitivity myocarditis or eosinophilic myocarditis. Histologically, this kind of myocarditis shows a clear interstitial infiltration of eosinophiles, in addition to lymphocytes, plasma cells and histiocytes, which mostly affect all sections of the heart in the sense of a pancarditis, often only with non-significant myocytolysis [27].
A further form of hypereosinophilic carditis is Löffler’s endocarditis. In this scenario, hypereosinophilia develops by various reasons and can cause multiorganic failure (heart, lung, borne marrow, brain). Mainly the endocardium is affected with biventricular endocardial thickening in the region of the outflow tract as well as in the region of the apex cordis [12].
Etiology of inflammatory cardiomyopathy/DCMi
Inflammatory cardiomyopathy (DCMi) is defined as a specific cardiomyopathy entity according to the report of the WHO/ISFC Task Force on the Definition and Classification of Cardiomyopathies 1995 [39, 56]. The diagnosis of DCMi is based on an exact analysis of EMBs using an arsenal of procedures, including histological, immunohistological, and virological tools (especially PCR). The pathophysiological concept of this disease entity is that myocardial inflammation and/or viral genomes may persist chronically due to an inadequate immune response after an acute viral induced myocarditis. This is confirmed by the fact that in patients with clinically suspected DCM with a chronic progression of LV dysfunction, viral persistence in the myocardium (Fig. 4) as well as myocardial inflammation (Fig. 5) can be detected in a high percentage in these patients [29, 31, 32, 47, 48, 50, 52]. In addition, this concept of transition from acute myocarditis to DCMi due to persiting low grade myocardial inflammation with or without viral persistence is confirmed by the following known effects. Viral persistence due to an inadequate immune response can cause direct cytopathic effects (i.e., cleavage of dystrophin by the enteroviral protease A2 [4]) as well as virus-induced anti-cardiac immune responses. If this immune response is uncontrolled, it can result in T-cell mediated anti-cardiac immunity, leading to progressive myocardial dysfunction and remodeling [47, 48]. Beside the cellular immunity, antibody linked immunity against myocardial proteins seems to be important [42, 43,45]. Autoantibodies against different myocardial antigens have been characterized. In addition, cytokines per se like tumor necrosis factor α (TNF-α), which have direct cardiodepressive effects, are elevated [41, 63]. Furthermore, they promote remodeling of the extracellular matrix by inducing an imbalance in the system of metalloproteinases and their inhibitors (tissue inhibitors of matrix metalloproteinases (TIMPs)) [37, 51, 54]. Therefore, all these effects causing a progressive destruction of the myocardium can finally lead to the clinical presentation of DCM. These pathogenic mechanisms involved in myocarditis and DCMi are summarized in Fig. 3.
Fig. 5.
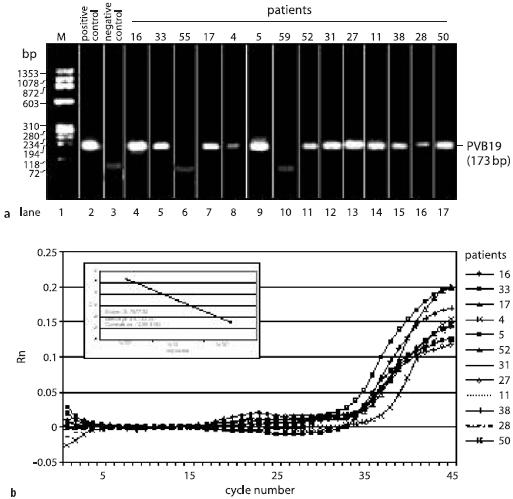
PCR, real-time PCR and sequence analysis of PVB19 in EMBs. a Qualitative proof of PVB19 genomes by nested PCR. b Quantitative assessment of PVB19 viral load by real-time PCR. Reproduced from [33]
Clinical course and presentation of acute myocarditis
The clinical course and presentation of acute myocarditis is highly variable. Presumably, a majority of the viral-induced cases pass in a clinically inapparent course. In some cases, however, fulminant courses with rapidly progressing LV dysfunction occur, which necessitates maximal intensive care therapy (i.e., inotropic support and implantation of an assist device) [21, 40, 53, 71]. Virus-caused impairment of systolic and diastolic LV function [64] are responsible for heart failure symptoms. Some patients may present with an acute coronary syndrome, including abrupt onset of angina, combined with ECG changes (ST-elevation or T-wave inversions) and increase of heart specific enzymes such as troponin or creatinin kinase [33], often linked to an antecedent flue-like illness period. Echocardiographic analysis often reveals a variable degree of LV dysfunction. A pericardial effusion (perimyocarditis) may be present during the acute stage of disease. A similar variability of complaints and spontaneous courses is also seen in non-infectious acute myocarditis [10].
Clinical course and presentation of inflammatory cardiomyopathy/DCMi
After the acute phase of the virus-induced acute myocarditis, approximately 20% of these patients develop a chronically progressive disease consistent with the clinical picture of DCM [13]. Because this transition is a highly dynamic course and not predictable, it is clinically impossible to decide, if acute myocarditis will heal, and whether intramyocardial inflammation or viral persistence will persist chronically in these patients. In addition, there are no clinical predictors that allow the identification of patients at risk of developing chronic progressive disease. So far, only by a detailed analysis of EMBs can a definitive diagnosis of DCMi (myocardial inflammation with or without viral persistence) be made, which has an impact on prognosis and initiation of rational treatment strategies (immunomodulation, antiviral) (Fig. 4).
Critical debate
PRO
Clinical parameters are not useful for the diagnosis of myocardial inflammation or virus persistence
Clinical presentation with pericardial effusion, ECG changes, elevation of cardiac specific enzymes (e.g., troponin-T, CK), or LV dysfunction in association with an antecedent viral infection may be considered as a relevant pathogen for the clinically suspected diagnosis of acute myocarditis, or acute myocarditis in the past, respectively. However, these clinical parameters are unspecific and do not substitute investigations of EMBs for myocardial inflammation or virus persistence, since clinical parameters do not correlate with myocardial inflammation with or without viral persistence or with the prognosis of the disease [32, 33]. In addition, the diagnosis of myocardial inflammation and/or viral persistence in patients with clinically suspected DCM is also impossible by using anamnestic or clinical data. However, the diagnosis of DCMi is based on the EMB proven detection of myocardial inflammation with or without viral persistence combined with an impairment of LV function. Therefore, a possible virus persistence or myocardial inflammation can only be substantiated by extensive examinations of EMBs in patients with clinically suspected myocarditis, myocarditis in the past and DCM. However, it should be emphasized that all other factors causing an impairment of LV function (e.g., coronary heart disease, significant valvular disease, severe arterial hypertension, and diabetes-induced heart failure) have to be excluded prior to EMB extraction.
Meaningful diagnosis of myocardial inflammation or viral persistence by a complete diagnostic arsenal (histology, immunohistology and virological analysis) in specially certified laboratories
Histology
Regarding myocardial inflammation, the histological examination of EMBs according to the Dallas classification is predominantly suitable to detect high grade inflammatory processes. According to the Dallas classification, these processes are defined as myocarditis (cellular infiltrates with myocyte necrosis with or without fibrosis) (Fig. 1) or borderline myocarditis (cellular infiltrates without myocyte necrosis) [3]. Under additional consideration of the follow-up EMB investigations and also the clinical course of the patients, ongoing, healing or healed myocarditis can be differentiated. Several studies have shown that the sole histological assessment of EMBs is unsuitable to detect especially low grade myocardial inflammation due to low sensitivity and specificity, sampling error and interobserver variability [24, 31, 48, 59]. This problem of sampling error is even enhanced in the acute stage of disease due to the often described focal pattern of inflammation. Hauck et al. demonstrated in post mortem tissue of patients with histologically proven lymphocytic myocarditis that with the evaluation of five biopsies, the histological diagnosis of myocarditis/borderline myocarditis showed false negative results in 55% of the cases, and the correct diagnosis could be confirmed only in 45% of the analyzed EMBs [24]. Therefore, the Dallas criteria based on histological analysis are not adequate to identify patients with viral or autoimmune-related myocardial dysfunction [5]. However, the histological analysis of EMBs is essential in the diagnosis of fibrosis, hypertrophy, and storage diseases. In addition, rare causes of myocarditis-like giant cell myocarditis or eosinophilic myocarditis can only be diagnosed by the histological analysis [1, 10, 11, 17, 21, 30]. Furthermore, other forms of non-infectious myocarditis, i.e., the involvement of the myocardium in connective tissue diseases or in sarcoidosis can only be verified by histological analysis in combination with special immunohistological stainings of EMBs [68].
Immunohistology
Using immunohistochemical techniques, it is possible to elucidate intramyocardial inflammation qualitatively and quantitatively with a high sensitivity and specificity compared to histological analysis according to the Dallas classification. Immunohistological evaluation offers the possibility of a phenotypic characterization of immunocompetent infiltrates such as lymphocytes and macrophages, as well as cytotoxic (perforin-positive) cells. These cytotoxic T-cells (CTL) are especially important since they are specifically involved in the T-cell mediated cardiomyocyte loss in DCMi [47]. Abundance of endothelial cell adhesion molecules (CAMs) is clearly detectable in EMBs with increased lymphocytic and macrophage infiltrates. There is a close association between CAMs expression and especially counter-receptor positive infiltrates, substantiating the pivotal role of endothelial CAMs expression for the transendothelial migration of circulating immunocompetent cells into the target organs [45]. In addition, the homogenous CAMs expression pattern in the myocardium (independent from the localization of infiltrating cells) helps to reduce the sampling error significantly, which implies that CAMs abundance per se can be regarded as a relevant diagnostic approach for DCMi even in the absence of infiltrates (Fig. 6) [26, 48, 69, 70]. Moreover, exact quantification of the myocardial inflammation can be carried out by digital image analysis systems in an observer-independent manner [44, 45]. This qualitative and quantitative analysis of the myocardial inflammatory process cannot be performed following a mere histological approach.
Fig. 6.
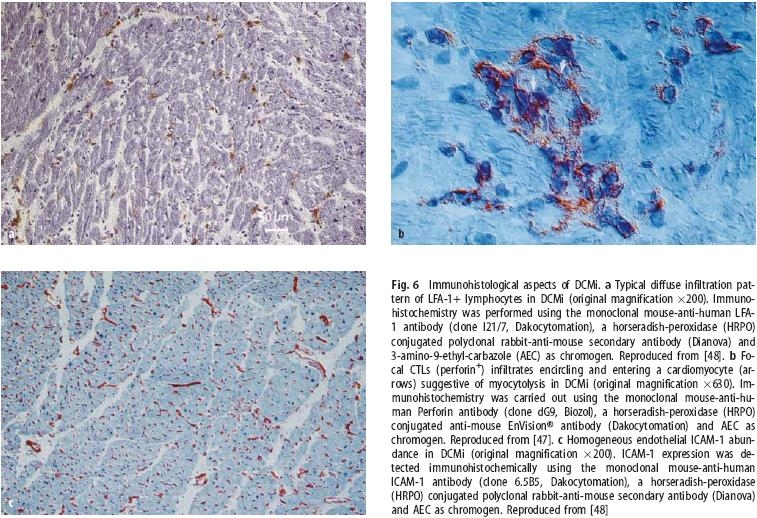
Immunohistological aspects of DCMi. a Typical diffuse infiltration pattern of LFA-1+ lymphocytes in DCMi (original magnification ×200). Immuno-histochemistry was performed using the monoclonal mouse-anti-human LFA-1 antibody (clone I21/7, Dakocytomation), a horseradish-peroxidase (HRPO) conjugated polyclonal rabbit-anti-mouse secondary antibody (Dianova) and 3-amino-9-ethyl-carbazole (AEC) as chromogen. Reproduced from [48]. b Focal CTLs (perforin+) infiltrates encircling and entering a cardiomyocyte (arrows) suggestive of myocytolysis in DCMi (original magnification ×630). Immunohistochemistry was carried out using the monoclonal mouse-anti-human Perforin antibody (clone dG9, Biozol), a horseradish-peroxidase (HRPO) conjugated anti-mouse EnVision® antibody (Dakocytomation) and AEC as chromogen. Reproduced from [47]. c Homogeneous endothelial ICAM-1 abundance in DCMi (original magnification ×200). ICAM-1 expression was detected immunohistochemically using the monoclonal mouse-anti-human ICAM-1 antibody (clone 6.5B5, Dakocytomation), a horseradish-peroxidase (HRPO) conjugated polyclonal rabbit-anti-mouse secondary antibody (Dianova) and AEC as chromogen. Reproduced from [48]
In conclusion, it should be pointed out that no techniques other than histology or immunohistology are suitable for the definitive diagnosis of myocardial inflammation in virus-induced as well as in noninfectious forms of myocarditis.
Virological analysis
Only by the use of molecular biological techniques (i.e., PCR, in situ hybridization) is it possible to detect reliably viral genomes in the myocardium of patients presenting with myocarditis or DCM [9, 29, 32, 33, 50, 52]. Apart from the mere presence of viral genomes, the biological activity of viruses (active replication versus viral latency) and the quantification of the virus load can be evaluated in EMBs solely by molecular biological methods [52, 64]. Concerning the analysis of biological activity, this evaluation is demonstrated so far only in enteroviral infection [52].
In general, the viral load of the myocardium has to be quantified by realtime PCR. The respective subtypes or cardiotropic viruses can be evaluated by sequence analysis. In the future, specific subtypes of cardiotropic viruses might gain special interest for the prognostic assessment of patients. Sequence analysis is also important as a method of quality assurance of the highly sensitive PCR technique, which is prone to contaminations (Fig. 5).
Other virological diagnostic techniques, i.e., serological investigations, are not useful in the context of myocardial virus persistence, since these analyses do not specify the organ afflicted by the tentative viral infection and moreover cannot distinguish biologically relevant myocardial infections from past infections. The latter point is of special interest, since cardiotropic viruses are ubiquitous, and past infections can be encountered frequently in adults. Therefore, this non-invasive approach is not suitable for the detection of a myocardial viral persistence. In addition, magnetic resonance imaging techniques are currently unable to detect virus genome in the myocardium. This approach is only helpful in diagnosing late enhancement as an indirect sign of myocardial inflammation and/or fibrosis [18, 28, 38].
Biopsy based causal therapy strategies and clinical relevance (histology, immunohistology, virological analysis)
Based on the new understanding of the pathogenesis of DCMi, causal treatment strategies have to be implemented. All studies concerning causal treatment strategies as well as clinical relevance of viral persistence or myocardial inflammation, respectively, must be based on EMB investigations. So far, all studies clearly substantiate the indispensable role of EMB diagnostics in this disease entity.
The prognostic relevance of enteroviral persistence was identified early in the study by Why et al. [67]. This study demonstrated that the mortality of patients with chronic impairment of LV function was significantly increased in the subgroup of patients with EMB proven diagnosis of myocardial persistence of enteroviral genome compared to virus negative patients (mortality: 25% versus 4%) over a mean observation period of 25 months (range, 11 to 50 months) [67]. In addition, a recent trial from Frustraci et al. showed clearly that in patients with viral persistence undergoing immunosuppressive therapy, the mortality was significantly increased [20]. On the other hand, the therapy concept of immunosuppression in patients with immunohistologically proven myocardial inflammation and exclusion of viral persistence diagnosed as DCMi demonstrated a significant improvement of LV dysfunction due to this causal EMB-based treatment strategy [69, 70].
This is additional evidence for the relevance of myocardial viral persistence for the course of the disease. There is a clear link between viral persistence and progression of LV dysfunction. In a recent study with a mean follow-up period of 6 months patients with spontaneous clearance of viral genomes (n=64 patients) demonstrated a significant improvement of LV function compared to patients with EMB proven persistence of viral genome in the myocardium (n=108 patients) [35].
Due to these investigations documenting the clinical relevance of viral persistence for disease progression, a pilot study was initiated to evaluate the beneficial effect of an antiviral treatment with interferon-β in patients with EMB proven persistence of adenoviral or enteroviral genomes in patients with chronic impairment of LV function. This pilot study demonstrated that this causal antiviral therapy strategy leads to a complete (100%) elimination of viral genomes, paralleled by a significant improvement of the clinical symptoms as well as a significant improvement of LV function [34]. In light of these very encouraging data, a prospective, multicenter, place-bo-controlled randomized phase II study (Betaferon® in Chronic Viral Cardiomyopathy — BICC Trial) with interferon-β was initiated to elucidate the therapeutic benefit of an antiviral therapy in patients with LV dysfunction and viral persistence. In the screening phase of this study all patients with chronic (> 6 months) left ventricular dysfunction (EF ≥30% to ≤55%) combined with persistent clinical symptoms of heart failure (NYHA II and NYHA III) after exclusion of coronary heart disease were enrolled. In addition, those patients were included into the treatment phase in whom enterovirus, adenovirus, or parvovirus B19 were detected and myocarditis according to the Dallas criteria in endomyocardial biopsies had been excluded.
The histological assessment of EMBs has a relevant prognostic and therapeutic impact only in non-infectious forms of acute myocarditis, like giant cell myocarditis or eosinophilc myocarditis. Without a histologically based diagnosis of giant cell myocarditis or eosinophilic myocarditis with a known highly decreased prognosis, implementation of immunosuppressive treatment strategies with a clearly documented positive effect is impossible.
The most profound data on the prognosis and specific treatment of this rare disease derive from the Giant Cell Myocarditis Registry with 63 patients. However, these data are derived from a registry, not a randomized controlled trial. The median time to death or cardiac transplantation for all patients was 5.5 months from the onset of symptoms. 70% of the patients died or required cardiac transplantation within 1 year, and the overall rate of death or cardiac transplantation was 89%. Treatment with combinations of immunosuppressive agents, but not corticosteroids alone, prolonged transplant-free survival significantly (p=0.001). Treatment with cyclosporine and steroids, occasionally combined with azathioprine and/or muromonab-CD3, was associated with a median survival of 12.6 months compared to 3.0 months compared to those not treated with immunosuppressive agents [11]. Cardiac transplantation remains an ultima ratio treatment option for giant cell myocarditis patients. However, recurrence of giant cell myocarditis has been reported. The 39 Giant Cell Myocarditis Registry patients who underwent heart transplantation had a 71% 5-year survival, despite a 25% post-transplantation recurrence rate by histologic EMB investigations [11]. Assist devices may be helpful to bridge giant cell myocarditis patients to transplantation, with a rate of successful bridging to transplantation in 78% of the patients, hence similar to that reported for other ventricular assist device recipients [1, 7].
Therefore, for a differentiated diagnostic procedure, the complete arsenal of EMB analyses including histology and immunohistology for the detailed diagnosis of myocardial inflammation and molecular biological techniques for the detection of viral persistence are mandatory for the etiopathogenic differentiation in patients with clinically suspected myocarditis in the past and clinically suspected DCM. In addition, causal treatment strategies can only be initiated after detailed analysis of EMBs concerning myocardial inflammation and viral persistence. Causal treatment strategies however should be still analyzed in controlled trials (e.g., Betaferon® in Chronic Viral Cardiomyopathy — BICC Trial).
Moreover, we have to point out that for a relevant diagnostic procedure at least six biopsies have to be taken in order to be able to perform the extensive histological, immunohistochemical and virological analyses in certified laboratories. This necessity of a relevant number of endomyocardial biopsies is due to the fact that a sampling error exists for the exact characterization of myocardial inflammation as well as viral persistence. To keep the risk of the procedure of taking biopsies as low as possible, it should only be performed in experienced centers.
Diagnosis of rare cases of myocardial affection of storage diseases
Mainly in cases with myocardial affection in the context of storage diseases (i.e., amyloidosis), specific histological investigations of EMBs are of decisive diagnostic and clinical importance (Table 1). Often, storage diseases show a diffuse pattern of myocardial affection, and therefore the histological assessment of the EMBs has a high sensitivity and specificity [2]. In addition, the storage diseases can be classified as a form of extracellular space infiltration between the myocytes (typically seen in amyloidosis) and a form infiltrating the myocytes (typically seen in lysosomal storage diseases (M. Fabry) and hemochromatosis). The exact diagnosis of myocardial storage disease is essential, because there are specific treatment options especially in Fabry’s disease [6]. This disease is caused by a deficit in the lysosomal enzyme α-galactosidase A (α-Gal A). Causal treatment options in Fabry’s disease are an enzyme enhancement therapy with galactose infusion [19] or an enzyme replacement therapy [15]. These treatment strategies have been effective in clearing glycolipid accumulation in myocytes, with an improvement in cardiac function [65]. Cardiac amyloidosis is characterized by extracellular amyloid infiltration throughout the heart. The presence of cardiac amyloidosis and its relative predominance varies with the type of amyloidosis. Five different types of amyloidosis have been described according to the underlying disease. These five forms are immunoglobulin amyloidosis, hereditary amyloidosis, senile systemic amyloidosis, secondary amyloidosis due to systemic inflammation, and hemodialysis-associated amyloidosis. Interestingly, secondary amyloidosis almost never affects the heart in any clinically significant manner. The common form of amyloidosis is the light-chain precursor amyloidosis (AL amyloidosis), which belongs to immunoglobulin amyloidosis. The cardiac involvement in light-chain precursor amyloidosis (AL amyloidosis) ranges from absent to severe infiltrative forms. AL amyloidosis is associated with a plasma cell dyscrasia which is related to multiple myeloma. Once congestive heart failure is present, the median survival is <6 months in untreated patients with AL amyloidosis. Therefore, early diagnosis and prompt initiation of therapy is essential in this disease entity. The final diagnosis of cardiac amyloidosis is based on the results of EMB analysis. The symptomatic therapy of cardiac AL amyloidosis is mainly based on high dose diuretics. ACE inhibitors and angiotensin II inhibitors are very poorly tolerated in subjects with AL amyloidosis. Even small doses may cause profound hypotension. Also β-blockers may be limited because of refractory heart failure or disease-related severe hypotension. Also digoxin is of little value in amyloidosis, and these patients may be at increased risk of digoxin toxicity because the drug binds avidly to amyloid fibrils. The definitive treatment of AL amyloidosis is antiplasma cell therapy aimed at stopping the production of the paraprotein responsible for the formation of amyloid [16]. This can be achieved by a number of chemotherapeutic regimens. In selected cases without progressive heart failure high dose chemotherapy with autologous stem cell replacement is also a considerable treatment option. However, in patients with advanced cardiac disease, peritreatment mortality is high (ca. 30%). An ejection fraction <40% is generally considered an absolute contraindication to high dose chemotherapy. In highly selected patients, cardiac transplantation may also be considered as a therapeutic approach. However, because patients with cardiac transplantation demonstrated a high long-term mortality due to disease progression in the heart and in noncardiac organs as well, a combined therapy of heart transplantation and high dose chemotherapy in combination with stem cell transplantation with the aim to abolish amyloid production seems to be a future therapeutic option.
Table 1.
Myocardial storage diseases
| Amyloidosis |
| Lysosomal storage disease (e.g., morbus Gaucher) |
| Glycogen storage disease (e.g., morbus Pompe) |
| Glycosphingolipid storage disease (morbus Fabry) |
| Mucopolysaccharidosis (e.g., morbus Hurler) |
| Hemochromatosis |
Less well known, and probably less common, is the cardiac manifestation of light-chain deposition disease (light-chain cardiomyopathy), which can mimic AL amyloidosis. The diagnosis of light-chain cardiomyopathy based on endomyocardial biopsies can be performed adequately only by using electron microscopy with antikappa or antilamda immunogold labeling. Concerning treatment options, chemotherapy targeting the underlying plasma cell dyscrasia may lead to reversal of the cardiomyopathy.
There are also a number of different hereditary forms of amyloidosis. In most cases, these hereditary forms of amyloidosis are due to the production of a mutant transthyretin protein in the liver. The onset of the disease occurs from the third decade on, most commonly after the age of 40. The deposition of transthyretin affects the myocardium as well as the conduction system. In addition, in some forms peripheral neuropathy may predominate. Since the abnormal transthyretin in hereditary amyloidosis is mainly produced by the liver, liver transplantation is the most important and the only definitive therapeutic intervention [16].
Contra
Lack of evidence based on phase III therapeutic studies which refer to the results of endomyocardial biopsy investigations
In patients with clinically suspected acute myocarditis, or histologically proven diagnosis of myocarditis/borderline myocarditis, respectively, no randomized phase III trial could demonstrate beneficial effects of immunosuppression or immunomodulatory treatment strategies as a causal EMB guided therapy option. The largest trial so far, the American Myocarditis Trial, in which patients with histologically proven myocarditis/borderline myocarditis according to the Dallas classification were enrolled without any analysis concerning the presence of myocardial viral persistence, did not show any beneficial effect of a causal immunosuppressive therapy in these patients [40].
In DCM patients with EMB proven myocardial inflammation and/or viral persistence, only small single center randomized trials or pilot trials, respectively, could demonstrate positive effects of causal treatment strategies (immunosuppression in patients with inflammation and exclusion of viral persistence; antiviral in patients with viral persistence) in this disease entity. Concerning the detection of viral persistence — especially of DNA viruses (human herpes virus type 6) — in the myocardium it has to be taken into account that the conformation of the viral genome in the myocardium by molecularbiological assays is not necessarily linked to active viral replication [8]. Therefore the interpretation of DNA virus persistence has to be handled with care. In addition, the results of a prospective, multicenter, placebocontrolled randomized study with interferon-β are still pending. One multicenter trial (Betaferon® in Chronic Viral Cardiomyopathy — BICC Trial) was initiated in order to elucidate the therapeutic benefit of antiviral therapy in patients with LV dysfunction and viral persistence. Depending on the results of this phase II trial, the need to perform EMBs in patients with viral persistence and progressive LV dysfunction has to be discussed. However, there is still no phase III trial proving any causal therapy concept [34, 36, 60, 69, 70].
Spontaneous complete healing of myocarditis
The transition from clinically suspected acute myocarditis or histologically proven myocarditis/borderline myocarditis, respectively, to a chronically progressive disease with progressive impairment of LV function is seen only in about 20% of the patients [13]. This unfavorable course is not predictable by the histological, immunohistochemical and virological findings of the EMB, and can only be evaluated after a close clinical follow-up concerning the detailed evaluation of clinical symptoms and heart function (e.g. echocardiography). In addition, the interpretation of EMB results in courses of spontaneous healing after an acute viral induced myocarditis has to be done very carefully, because in this transition period between acute infection and healing nonfeasible results may be produced, which have to be controlled in the follow-up period. Therefore the indication for EMB in patients with clinically suspected acute myocarditis has to be weighed on a case-by-case basis. However, especially in patients with rapid progressive impairment of LV function despite complete conventional heart failure treatment, in these cases EMB investigations should be performed to verify the clinically suspected diagnosis of acute viral myocarditis or to rule out rare cases of acute myocarditis, e.g., giant cell myocarditis [7, 10, 11, 14, 23] with good treatment options [11].
Age-related limitations for endomyocardial biopsy investigations
In light of the lack of robust data on the diagnostic and clinical value of EMB diagnostics in the elderly (i.e., >75 years), on the one hand, and due to the limited life expectancy of these patients, as well as the increasing complexity of polimorbidity often occurring in the elderly, EMB obtainment for diagnostic use has to be considered very carefully, although myocarditis seems to be quite frequent in elderly patients. In addition, performing EMBs in the elderly is technically not more difficult than in younger patients. However, the occurrence of concomitant diseases as well as the clinical condition of the elderly patient has to be considered before deciding to perform EMB investigations.
Conclusions
In patients with clinically suspected acute myocarditis and progression of disease despite complete heart failure medication, EMB investigations are indicated. This approach is necessary for the verification of the clinically suspected diagnosis of acute viral myocarditis as well as for the diagnosis of rare pathogenic entities of acute myocarditis (i.e., giant-cell myocarditis, hypersensitivity myocarditis, eosinophilic myocarditis) aiming at specific treatment strategies (e.g., immunosuppression) at this very early stage of the disease.
In patients with suspected myocarditis in the past or DCM, EMB investigations are indicated provided that the complete arsenal of contemporary EMB analyses are performed in certified laboratories and if a progressive deterioration of LV function and/or progression of heart failure symptoms despite complete heart failure medication is documented. Contemporary EMB analyses include histological and immunohistological analysis for the detailed diagnosis of myocardial inflammation, and molecular biological techniques for the detection of viral persistence including quantification of the virus load and sequence analysis of the amplified viral genomes. Only this approach enables the elucidation of the underlying pathogenesis and the introduction of causal treatment modalities, since they strongly depend on the EMB-based differential diagnosis (Fig. 4).
Acknowledgement
This work was supported by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich Transregio 19).
Abbreviations
- BICC-Trial
Betaferon® in Chronic Viral Cardiomyopathy
- CK
Creatinine kinase
- DCM
Dilated cardiomyopathy
- DCMi
Inflammatory cardiomyopathy
- ECG
Electrocardiogram
- EMB
Endomyocardial biopsy
- LV
Left ventricle
- PCR
Polymerase chain reaction
- TIMP
Tissue inhibitors of matrix metalloproteinases
References
- 1.Abril A, Calamia KT, Cohen MD (2003) The Churg Strauss syndrome: review and update. Semin Arthritis Rheum 33:106-14 [DOI] [PubMed]
- 2.Ardehali H, Qasim A, Capolla T, Howard D, Hruban R, Hare JM, Baughman KL, Kasper EK (2004) Endomyocardial biopsy plays a role in diagnosing patients with unexplained cardiomyopathy. Am Heart J 147:919-23 [DOI] [PubMed]
- 3.Aretz HT, Billingham ME, Edwards WD, Factor SM, fallon JT, Fenoglio JJ Jr, Olsen EG, Schoen FJ (1987) Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol 1(1):3-4 [PubMed]
- 4.Badorff C, Lee GH, Lamphear BJ, Martone ME, Campbell KP, Rhoads RE, Knowlton KU (1999) Enteroviral protease 2A cleaves dystrophin: evidence of cytoskeletal disruption in an acquired cardiomyopathy. Nat Med 5:320-26 [DOI] [PubMed]
- 5.Baughman KL (2006) Diagnosis of myocarditis: death of Dallas criteria. Circulation 113:593-95 [DOI] [PubMed]
- 6.Beer G, Reinecke P, Gabbert HE, Hort W, Kuhn H (2002) Fabry disease in patients with hypertrophic cardiomyopathy (HCM). Z Kardiol 91(12):992-002 [DOI] [PubMed]
- 7.Brilakis ES, Olson LJ, Berry GJ, Daly RC, Loisance D, Zucker M, Cooper LT Jr (2000) Survival outcomes of patients with giant cell myocarditis bridged by ventricular assist devices. Asaio J 46:569-72 [DOI] [PubMed]
- 8.Bonnafous P, Gautheret-Dejean A, Boutolleau D, Caiola D, Agut H (2005) Persistence of DNA in cell cultures may jeopardize the analysis of human herpesvirus 6 dynamics by means of real-time PCR. J Virol Methods 125(1):95-8 [DOI] [PubMed]
- 9.Bowles NE, Ni J, Kearney DL (2003) Detection of viruses in myocardial tissues by polymerase chain reaction evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol 42(3):466-472 [DOI] [PubMed]
- 10.Cooper LT (2000) Giant cell myocarditis: diagnosis and treatment. Herz 25:291-98 [DOI] [PubMed]
- 11.Cooper LT, Berry GJ, Shabetai R (1997) Idiopathic giant-cell-myocarditis-natural history and treatment. Multicenter Giant Cell Myocarditis Study Group Investigators. N Engl J Med 336(26):1860-866 [DOI] [PubMed]
- 12.Corssmit EP, Trip MD, Durrer JD (1999) Loffler’s endomyocarditis in the ideopathic hypereosinophilic syndrome. Cardiology 91(4):272-76 [DOI] [PubMed]
- 13.D’Ambrosio A, Patti G, Manzoli A, Sinagra G, Di Lenarda A, Silvestri F, Di Sciascio G (2001) The fate of acute myocarditis between spontaneous improvement and evolution to dilated cardiomyopathy: a review. Heart 85:499-04 [DOI] [PMC free article] [PubMed]
- 14.Davies RA, Veinot JP, Smith S, Struthers C, Hendry P, Masters R (2002) Giant cell myocarditis: clinical presentation, bridge to transplantation with mechanical circulatory support, and long-term outcome. J Heart Lung Transplant 21:674-79 [DOI] [PubMed]
- 15.Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldek S, Caplan L, Linthorst GE, Desnick RJ; International Collaborative Fabry Disease Study Group (2001) Safety and efficacy of recombinant human alpha-galactosidase. A replacement therapy in Fabry’s disease. New Engl J Med 345:9-6 [DOI] [PubMed]
- 16.Falk RH (2005) Diagnosis and management of the cardiac amyloidosis. Circulation 112:2047-060 [DOI] [PubMed]
- 17.Fenoglio JJ, McAllister HA, Mullick FG (1981) Drug related myocarditis. I. Hypersensitivity myocarditis. Hum Pathol 12:900-07 [DOI] [PubMed]
- 18.Friedrich MG, Strohm O, Schulz- Menger J, Marciniak H, Luft FC, Dietz R (1998) Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation 97:1802-809 [DOI] [PubMed]
- 19.Frustaci A, Chimenti C, Ricci R, Natale L, Russo MA, Pieroni M, Eng CM, Desnick RJ (2001) Improvement in cardiac function in the cardiac variant of Fabry’s disease with galactose- infusion therapy. New Engl J Med 345:25-2 [DOI] [PubMed]
- 20.Frustaci A, Chimenti C, Calabrese F, Pieroni M, Thiene G, Maseri A (2003) Immunosuppressive therapy for active lymphocytic myocarditis: virologic and immunologic profile of responders versus non-responders. Circulation 107:857-63 [DOI] [PubMed]
- 21.Grabellus F, Hoffmeier A, Schmitz KJ, Kandolf R, Bultmann BD, Scheld HH, Baba HA (2003) Resolved hypertensitivity myocarditis after ventricular circulatory assist. Ann Thorac Surg 76(6):2102-104 [DOI] [PubMed]
- 22.Grogan M, Redfield MM, Bailey KR (1995) Long-term outcome of patients with biopsy-proved myocarditis: comparison with idiopathic dilated cardiomyopathy. J Am Coll Cardiol 26:80-4 [DOI] [PubMed]
- 23.Haas GJ (2001) Etiology, evaluation, and management of acute myocarditis. Cardiol Rev 9(2):88-5 [DOI] [PubMed]
- 24.Hauck AJ, Kearney DL, Edwards WD (1989) Evaluation of postmortem endomyocardial biopsy specimens from 38 patients with lymphocytic myocarditis: implications for role of sampling error. Mayo Clin Proc 64:1235-245 [DOI] [PubMed]
- 25.Hengge UR, Tannapfel A, Tyring SK, Erbel R, Arendt G, Ruzicka T (2003) Lyme borreliosis. Lancet Infect Dis 3(8):489-00 [DOI] [PubMed]
- 26.Herskowitz A, Ahmed-Ansari A, Neumann DA, Beschorner WE, Rose NR, Soule LM, Burek CL, Sell KW, Baughman KL (1990) Induction of major histocompatibility complex antigens within the myocardium of patients with active myocarditis: a nonhistologic marker of myocarditis. J Am Coll Cardiol 15:624-32 [DOI] [PubMed]
- 27.Huntgeburth M, Lindner M, Fries JWU, Hoppe UC (2005) Hypereosinophilic syndrome associated with acute necrotizing myocarditis and cardiomyopathy. Z Kardiol 94:761-66 [DOI] [PubMed]
- 28.Kadalie CT (2005) Stellenwert der MRT bei chronischer Myokarditis. Z Kardiol 94(Suppl 4):IV94–IV96 [DOI] [PubMed]
- 29.Klein RM, Jiang H, Niedracher D, Adams O, Du M, Horlitz M, Schley P, Marx R, Lankisch MR, Brehm MU, Strauer BE, Gabbert HE, Scheffold T, Gülker H (2004) Freqneuncy and quantity of the parvovirus B19 genome in endomyocardial biopsies from patients with suspected myocarditis or idiopathic left ventricular dysfunction. Z Kardiol 93(4):300-09 [DOI] [PubMed]
- 30.Konius NG, Zavras GM, Soufras GD, Kitrou MP (1989) Hypersensitivity myocarditis. Ann Allergy 62:71-4 [PubMed]
- 31.Kuhl U, Noutsias M, Seeberg B, Schultheiss HP (1996) Immunohistological evidence for a chronic intramyocardial inflammatory process in dilated cardiomyopathy. Heart 75:295-00 [DOI] [PMC free article] [PubMed]
- 32.Kuhl U, Pauschinger M, Noutsias M, Seeberg B, Bock T, Lassner D, Poller W, Kandolf R, Schultheiss HP (2005) High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “idiopathic-left ventricular dysfunction. Circulation 111(7):887-93 [DOI] [PubMed]
- 33.Kuhl U, Pauschinger M, Bock T, Klingel K, Schwimmbeck CP, Seeberg B, Krautwurm L, Poller W, Schultheiss HP, Kandolf R (2003) Parvovirus B19 infection mimicking acute myocardial infarction. Circulation 108:945-50 [DOI] [PubMed]
- 34.Kuhl U, Pauschinger M, Schwimmbeck PL, Seeberg B, Lober C, Noutsias M, Poller W, Schultheiss HP (2003) Interferon-beta treatment eliminates cardiotropic viruses and improves left ventricular function in patients with myocardial persistence of viral genomes and left ventricular dysfunction. Circulation 107:2793-2798 [DOI] [PubMed]
- 35.Kuhl U, Seeberg B, Noutsias M, Poller W, Schultheiss HP, Pauschinger M (2005) Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation 112(13):1965-970 [DOI] [PubMed]
- 36.Kuhl U, Schultheiss HP (1995) Treatment of chronic myocarditis with corticosteroids. Eur Heart J 16:168-72 [DOI] [PubMed]
- 37.Li J, Schwimmbeck PL, Tschope C, Leschka S, Husmann L, Rutschow S, Reichenbach F, Noutsias M, Kobalz U, Poller W, Spillmann F, Zeichhardt H, Schultheiss HP, Pauschinger M (2002) Collagen degradation in a murine myocarditis model: relevance of matrix metalloproteinase in association with inflammatory induction. Cardiovasc Res 56:235-47 [DOI] [PubMed]
- 38.Marholdt H, Goedecke C, Wagner A, Meinhardt G, Athanasiadis A, Vogelsberg H, Fritz P, Klingel K, Kandolf R, Sechtem U (2004) Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation 109:1250-258 [DOI] [PubMed]
- 39.Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB (2006) Contemporary definitions and classification of the cardiomyopathies: An American Heart Association scientific statement from the council on clinical cardiology, heart failure and transplantation committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and council on epidemiology, and prevention. Circulation 113:1807-1816 [DOI] [PubMed]
- 40.Mason JW, O’Connell JB, Herskowitz A (1995) A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N Engl J Med 333:269-75 [DOI] [PubMed]
- 41.Matsumori A (1996) Cytokines in myocarditis and cardiomyopathies. Curr Opin Cardiol 11:302-09 [DOI] [PubMed]
- 42.McCarthy RE 3rd, Boehmer JP, Hruban RH, Hutchins GM, Kasper EK, Hare JM, Baughman KL (2000) Longterm outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N Engl J Med 342(10):690-95 [DOI] [PubMed]
- 43.Mues B, Brisse B, Zwadlo G, Themann H, Bender F, Sorg C (1990) Phenotyping of macrophages with monoclonal antibodies in endomyocardial biopsies as a new approach to diagnosis of myocarditis. Eur Heart J 11:619-27 [DOI] [PubMed]
- 44.Noutsias M, Fechner H, de Jonge H, Wang X, Dekkers D, Houtsmuller AB, Pauschinger M, Bergelson J, Warraich R, Yacoub M, Hetzer R, Lamers J, Schultheiss HP, Poller W (2001) Human coxsackie-adenovirus receptor is colocalized with integrins alpha(v) beta(3) and alpha(v)beta(5) on the cardiomyocyte sarcolemma and upregulated in dilated cardiomyopathy: implications for cardiotropic viral infections. Circulation 104:275-80 [DOI] [PubMed]
- 45.Noutsias M, Pauschinger M, Ostermann K, Escher F, Blohm JH, Schultheiss H, Kuhl U (2002) Digital image analysis system for the quantification of infiltrates and cell adhesion molecules in inflammatory cardiomyopathy. Med Sci Monit 8:MT59-1 [PubMed]
- 46.Noutsias M, Pauschinger M, Schultheiss H, Kuhl U (2002) Phenotypic characterization of infiltrates in dilated cardiomyopathy -diagnostic significance of T-lymphocytes and macrophages in inflammatory cardiomyopathy. Med Sci Monit 8:CR478-487 [PubMed]
- 47.Noutsias M, Pauschinger M, Schultheiss HP, Kuhl U (2003) Cytotoxic perforin+ and TIA-1+ infiltrates are associated with cell adhesion molecule expression in dilated cardiomyopathy. Eur J Heart Fail 5:469-79 [DOI] [PubMed]
- 48.Noutsias M, Seeberg B, Schultheiss HP, Kuhl U (1999) Expression of cell adhesion molecules in dilated cardiomyopathy: evidence for endothelial activation in inflammatory cardiomyopathy. Circulation 99:2124-131 [DOI] [PubMed]
- 49.Okura Y, Dec GW, Hare JM, Kodama M, Berry GJ, Tazelaar HD, Bailey KR, Cooper LT (2003) A clinical and histopathologic comparison of cardiac sarcoidosis and idiopathic giant cell myocarditis. J Am Coll Cardiol 41(2):322-29 [DOI] [PubMed]
- 50.Pauschinger M, Bowles NE, Fuentes- Garcia FJ, Pham V, Kuhl U, Schwimmbeck PL, Schultheiss HP, Towbin JA (1999) Detection of adenoviral genome in the myocardium of adult patients with idiopathic left ventricular dysfunction. Circulation 99:1348-354 [DOI] [PubMed]
- 51.Pauschinger M, Chandrasekharan K, Schultheiss H-P (2004) Myocardial remodeling in viral heart disease: Possible interactions between inflammatory mediators and MMP-TIMP system. Heart Fail Rev 9:21-1 [DOI] [PubMed]
- 52.Pauschinger M, Doerner A, Kuhl U, Schwimmbeck PL, Poller W, Kandolf R, Schultheiss HP (1999) Enteroviral RNA replication in the myocardium of patients with left ventricular dysfunction and clinically suspected myocarditis. Circulation 99:889-95 [DOI] [PubMed]
- 53.Pauschinger M, Noutsias M, Rutschow S, Kuhl U, Schultheiss H-P (2005) Acute myocarditis -diagnosis and therapeutical options. Intensivund Notfallbehandlung 30:19-9
- 54.Pauschinger M, Rutschow S, Chandrasekharan K, Westermann D, Weitz A, Schwimmbeck PL, Zeichhardt H, Poller W, Noutsias M, Li J, Schultheiss H-P, Tschoepe C (2005) Carvedilol improves left ventricular function in murine coxsackievirus-induced acute myocarditis Association with reduced myocardial interleukin 1β and MMP-8 expression. Eur J Heart Failure 7(4):444-52 [DOI] [PubMed]
- 55.Poller W, Fechner H, Noutsias M, Tschoepe C, Schultheiss H-P (2002) Highly variable expression of virus receptors in the human cardiovascular system -Implications for cardiotropic viral infections and gene therapy. Z Kardiol 91:978-91 [DOI] [PubMed]
- 56.Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O’Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P (1996) Report of the 1995 World Health Organization/ International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 93:841-42 [DOI] [PubMed]
- 57.Schultheiss HP, Kuhl U, Janda I, Melzner B, Ulrich G, Morad M (1988) Antibody-mediated enhancement of calcium permeability in cardiac myocytes. J Exp Med 168:2105-119 [DOI] [PMC free article] [PubMed]
- 58.Schulze K, Becker BF, Schauer R, Schultheiss HP (1990) Antibodies to ADP-ATP carrier -an autoantigen in myocarditis and dilated cardiomyopathy -impair cardiac function. Circulation 81:959-69 [DOI] [PubMed]
- 59.Shanes JG, Ghali J, Billingham ME, Ferrans VJ, Fenoglio JJ, Edwards WD, Tsai CC, Saffitz JE, Isner J, Furner S (1987) Interobserver variability in the pathologic interpretation of endomyocardial biopsy results. Circulation 75:401-05 [DOI] [PubMed]
- 60.Staudt A, Schaper F, Stangl V, Plagemann A, Bohm M, Merkel K, Wallukat G, Wernecke KD, Stangl K, Baumann G, Felix SB (2001) Immunohistological changes in dilated cardiomyopathy induced by immunoadsorption therapy and subsequent immunoglobulin substitution. Circulation 103:2681-686 [DOI] [PubMed]
- 61.Staudt A, Staudt Y, Dorr M, Bohm M, Knebel F, Hummel A, Wunderle L, Tiburcy M, Wernecke KD, Baumann G, Felix SB (2004) Potential role of humoral immunity in cardiac dysfunction of patients suffering from dilated cardiomyopathy. J Am Coll Cardiol 44:829-36 [DOI] [PubMed]
- 62.Tanowitz HB, Kirchhoff LV, Simon D, Morris SA, Weiss LM, Wittner M (1992) Chagas-disease. Clin Microbiol Rev 5:400-19 [DOI] [PMC free article] [PubMed]
- 63.Torre-Amione G, Kapadia S, Lee J, Durand JB, Bies RD, Young JB, Mann DL (1996) Tumor necrosis factor-alpha and tumor necrosis factor receptors in the failing human heart. Circulation 93(4):704-11 [DOI] [PubMed]
- 64.Tschope C, Bock CT, Kasner M, Noutsias M, Westermann D, Schwimmbeck PL, Pauschinger M, Poller WC, Kuhl U, Kandolf R, Schultheiss HP (2005) High prevalence of cardiac parvovirus B19 infection in patients with isolated left ventricular diastolic dysfunction. Circulation 111:879-86 [DOI] [PubMed]
- 65.Weidemann F, Breunig F, Beer M et al (2003) Improvement of cardiac function during enzyme replacement therapy in patients with Fabry disease: a prospective strain rate imaging study. Circulation 108:1299-301 [DOI] [PubMed]
- 66.Wessely R, Klingel K, Santana LF, Dalton N, Hongo M, Jonathan Lederer W, Kandolf R, Knowlton KU (1998) Transgenic expression of replication- restricted enteroviral genomes in heart muscle induces defective excitation-contraction coupling and dilated cardiomyopathy. J Clin Invest 102(7):1444-453 [DOI] [PMC free article] [PubMed]
- 67.Why HJ, Meany BT, Richardson PJ (1994) Clinical and prognostic significance of detection of enteroviral RNA in the myocardium of patients with myocarditis or dilated cardiomyopathy. Circulation 89:2582-589 [DOI] [PubMed]
- 68.Wijetunga M, Rockson S (2002) Myocarditis in systemic lupus erythematosus. Am J Med 113(5):419-23 [DOI] [PubMed]
- 69.Wojnicz R, Nowalany-Kozielska E, Wojciechowska C (2001) Randomized, placebo-controlled study for immunosuppressive treatment of inflammatory dilated cardiomyopathy: two-year follow-up results. Circulation 104:39-5 [DOI] [PubMed]
- 70.Wojnicz R, Nowalany-Kozielska E, Wojciechowska C, Glanowska G, Wilczewski P, Niklewski T, Zembala M, Polo, nacute, ski L, Rozek MM, Wodniecki J (2001) Randomized, placebo- controlled study for immunosuppressive treatment of inflammatory dilated cardiomyopathy: twoyear follow-up results. Circulation 104:39-5 [DOI] [PubMed]
- 71.Yacoub MH (2001) A novel strategy to maximize the efficacy of left ventricular assist devices as a bridge to recovery. Eur Heart J 22:534-40 [DOI] [PubMed]


