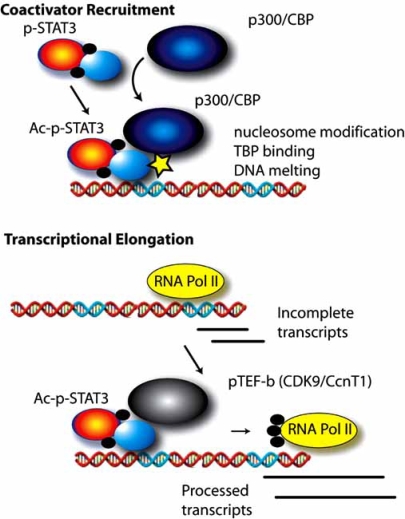
Fig. (2).
Discrete mechanisms for IL-6 induction of target genes. Top, coactivator recruitment mechanism. Tyrosine phosphorylated STAT3 binds to p300/CBP, resulting in STAT3 acetylation (Ac) on its NH2 terminus, and stabilization of the STAT3-p300/CBP complex. The acetylated-phosphorylated STAT3-p300/CBP complex then binds to high affinity IL-6 response elements in the promoters of target genes. This complex induces nucleosomal reorganization via the p300 histone acetylase activity, pre-initiation complex formation, recruiting TATA box binding protein, and enhanced RNA polymerase II activity. Bottom, transcriptional elongation. In a subset of IL-6 responsive promoters, RNA polymerase (Pol) II is engaged with the promoter producing incomplete transcripts. During the process of activation, tyrosine phosphorylated STAT3 complexes with the positive transcriptional elongation factor (PTEF-b), a complex containing CDK9. CDK9 phosphorylates the COOH terminal domain of RNA polymerase II, enabling it to enter productive elongation mode, producing full length RNA transcripts.