Abstract
Cardiovascular disease (CVD) and osteoporosis are common age-related conditions associated with significant morbidity, mortality, and disability.
Traditionally, these two conditions were considered unrelated and their coexistence was attributed to independent age-related processes. However, an increasing body of biological and epidemiological evidence has provided support for a link between the two conditions that cannot be explained by age alone. Several hypotheses have been proposed to explain the link between osteoporosis and CVD including: 1) shared risk factors, 2) common pathophysiological mechanisms, 3) common genetic factors, or 4) a causal association.
This review highlights the epidemiologic literature on the association of bone density with cardiovascular mortality, cardiovascular morbidity, and subclinical measures of atherosclerosis. It also summarizes the different potential mechanisms involved in the link between osteoporosis and CVD.
Keywords: osteoporosis, bone mineral density, cardiovascular disease, atherosclerosis, vascular calcification
Osteoporosis and cardiovascular disease
Cardiovascular disease (CVD) and osteoporosis are common age-related conditions. Mounting biological and epidemiological evidence supports a link between the two diseases. Low bone mineral density (BMD) has been related to increased cardiovascular mortality, cardiovascular morbidity, and subclinical measures of atherosclerosis in cross-sectional as well as longitudinal epidemiologic studies.
Biological link
Atherosclerotic calcification and bone mineralization share a number of intriguing common features. It is now recognized that calcification of the arterial tissue is not merely a passive process of calcium phosphate precipitation or adsorption in end-stage atherosclerosis, but instead is a highly organized process that is regulated by mechanisms similar to those involved in bone mineralization (1, 2).
The mineral observed in calcium deposits of atherosclerotic plaques has a very similar chemical composition to hydroxyapatite crystals which form the inorganic bone matrix (3, 4). Calcifiable vesicles were isolated from human atherosclerotic aortas (5), suggesting that these may be involved in mineral deposition, similar to “extracellular matrix vesicles” that are secreted from chondrocytes and osteoblasts and are involved in initial bone mineralization. Calcified plaques were also shown to express several bone matrix proteins such as type I collagen, gla (gamma carboxyglutamate)-containing proteins such as osteocalcin (bone-gla protein) and matrix-gla protein, bone morphogenetic protein (BMP)-2 and -4, osteopontin, osteonectin, and bone sialoprotein (3, 6-8). Osteogenic cells, called calcifying vascular cells (CVCs), were identified in atherosclerotic plaques. These are a subpopulation of vascular smooth muscle cells (VSMC) that are capable of osteoblastic differentiation (3, 9). When stimulated by BMP-2 and BMP-4, these cells begin expressing osteoblast genes including alkaline phosphatase, collagen I, and osteocalcin which are needed for bone formation. Other cells involved in bone metabolism including osteoclast-like cells, chondrocyte-like cells, and hematopoietic bone marrow cells were also seen in plaques (10).
Epidemiologic link
Bone mass and cardiovascular mortality
Low BMD and bone loss appear to be risk factors for cardiovascular mortality in both women (11-14) and men (15, 16) (TableI). The Study of Osteoporotic Fractures (SOF) showed that an increase in BMD loss at the hip in the order of one standard deviation (SD) was associated with a 1.3-fold increase in CHD mortality among white women 65 years of age and older. Similarly, calcaneal bone loss was related to increased risk of death due to atherosclerosis [Relative Risk1(RR = 1.2, 95% CI = 1.1-1.4) and CHD (RR = 1.3, 95% CI = 1.0-1.6)] (12). In the same cohort, lower broadband ultrasound attenuation (BUA) and calcaneal BMD were related to higher cardiovascular death (11), and decreased BMD of the proximal radius was related to increased risk of stroke mortality (RR = 1.91, 95% CI 1.25-2.92) (14). In a population of Danish women, low bone mineral content in the forearm at the menopause was associated with an increased cardiovascular death later in life (RR = 2.3 per SD decrease in BMD, 95% CI 1.0-4.9). In the same study, a prevalent vertebral compression fracture was independently associated with cardiovascular death in late postmenopausal women (RR = 2.0, 95%CI 1.4-3.3) (13).
Table I -.
Summary of epidemiologic studies of BMD and cardiovascular mortality.
| Author | Design | Study | Population | BMD measurement | Mortality | Result | Comment |
|---|---|---|---|---|---|---|---|
| Mussolino et al., 2003 | Prospective (median follow-up= 18.5 years) | NHANES I Epidemiologic Follow-up Study | White and black, men and women, 45-74 years, n=3501 | Phalangeal BMD (RA) | Mortality (total, cardiovascular, non-cardiovascular) |
|
Adjusted for age, smoking, alcohol, diabetes, heart disease, education, BMI, physical activity and blood pressure medications |
| Mussolino et al., 2003 | Prospective | NHANES I | White and black, men and women, 45-74 years, n=3402 | Phalangeal BMD (RA) | Stroke mortality | No association between BMD and stroke mortality | Adjusted for age, smoking, alcohol consumption, history of diabetes, history of heart disease, education, BMI, physical activity, and blood pressure medications |
| Bauer et al., 2002 | Prospective (average follow-up= 5 years) | SOF | White, postmenopausa women, 70 years l and older, n= 5816 |
|
Total and cause specific mortality(CVD, cancer) |
|
Adjusted for age, weight, height, smoking, physical activity, history of diabetes, hypertension, cancer, CVD, and stroke |
| Trivedi et al., 2001 | Prospective (average follow-up= 6.7 years) | The Cambridge General Practice Health Study | White men, 65-76 years, n= 1002 | Total hip BMD (DXA) | Mortality (all-cause, cardiovascular) |
|
Adjusted for age, BMI, smoking, cholesterol, SBP, past history of MI, stroke, or cancer, physical activity, alcohol, and general health status |
| Kado et al., 2000 | Prospective (average follow-up= 3.2 years) | SOF | White, postmenopausal women, 65 years and older, n= 6046 |
|
Mortality (CHD, stroke, atherosclerosis, cancer , all other causes) |
|
Adjusted for age, baseline BMD, diabetes, hypertension, incident fractures, smoking, physical activity, health status, weight loss, calcium use |
| von der Recke et al., 1999 | Retrospective cohort | Danish Study | White, early postmenopausal (5,216 years of follow-up) and late postmenopausal (6,292 years of follow-up) women, n= 1,063 |
|
Mortality (cerebrovascular disease, heart disease, vascular disease, cancer) |
|
Adjusted for age, systolic blood pressure, diastolic blood pressure, BMI, cholesterol levels, smoking |
| Browner et al., 1991 | Prospective (average follow-up= 2.8 years) | SOF | White, postmenopausal women, 65 years and older (n= 9704) | Distal radius, proximal radius, and calcaneal BMD (SPA) | Mortality (all-cause, stroke) |
|
Stroke mortality: adjusted for history of previous stroke, hypertension, postmenopausal use of estrogen, thiazide diuretic treatment, diabetes mellitus, and smoking |
Similar results were observed in men. Results from the NHANES I Epidemiologic Follow-up Study indicated that low phalangeal BMD was a significant predictor of subsequent cardiovascular mortality among white men aged 45 to 74 years (RR = 1.16, 95% CI 1.0-1.30). This association, however, was not present in white women or blacks (15). In another prospective study, low bone density at the hip was found to be a significant predictor of cardiovascular mortality in a cohort of British men aged 65-76 years (16).
Contrary to the above studies, Mussolino et al. did not find a significant association between BMD and stroke mortality in white men, white women, or blacks in NHANES I (17).
Bone mass and cardiovascular morbidity
A number of studies have investigated the association between BMD and cardiovascular morbidity (TableII). In a cross-sectional analysis from the Health, Aging, and Body Composition (ABC) Study, we observed that volumetric BMD (vBMD) measures of the spine were significantly and inversely associated with prevalent CVD in men and women, and areal BMD (aB-MD) of the trochanter was related to CVD in women (18). In a longitudinal analysis from the same cohort, we found that vB-MD measures of the spine were associated with incident CVD in white men, but not in blacks. In women, aBMD measures of the total hip, femoral neck, and trochanter exhibited significant relationships with incident CVD in black women, but not in whites. All of these associations were independent of age and shared risk factors between osteoporosis and CVD, and were not explained by inflammatory cytokines or oxidized LDL (TablesIIIandIV) (19).
Table II -.
Summary of epidemiologic studies of BMD and cardiovascular morbidity.
| Author | Design | Study | Population | BMD measurement | CVD endpoint | Result | Comment |
|---|---|---|---|---|---|---|---|
| Farhat et al., 2007 | Prospective (average follow-up of 5.4 years) | Health ABC | 2,310 participants, 55% women, 42% black, aged 68-80 years |
|
Incident CHD, cerebrovascular disease, or carotid artery disease |
|
– |
| Farhat et al., 2006 | Cross-sectional | Health ABC | 3,075 participants, 51% women, 42% black |
|
Prevalent CVD (CHD, peripheral arterial disease, cerebrovascular disease congestive heart failure) |
|
– |
| Tanko et al., 2005 | Prospective (4-years follow-up) | MORE Study | 2,576 postmenopausal women assigned to the placebo arm of the MORE trial, mean age= 66.5 years. |
|
Incidence of fatal and non-fatal cardiovascular events (coronary events and cerebrovascular events) |
|
|
| Magnus et al., 2005 | Cross-sectional | NHANES III | 5,050 African-American, Mexican-American, and Caucasian men and women. Aged 50-79 years | Total hip BMD (DXA) | Myocardial infarction |
|
|
| Marcovitz et al., 2005 | Retrospective | Ambulatory adult patients | 209 patients, 89% women, 91% white, average age= 67 years | Spine, femur, ultradistal radius, and 1/3 distal radius (DXA) | Angiographicallydetermined coronary artery disease (=50% luminal narrowing in a major artery) |
|
|
| Samelson et al., 2004 | Prospective (30year follow-up) | The Framingham Study | White, men and women, 47-80 years, (n= 2,059) | Relative metacarpal cortical area (Radiogrammetry) | Incident CHD |
|
Adjusted for age, education, BMI, smoking, alcohol, systolic blood pressure, cholesterol, HDL, and diabetes |
| Jørgensen et al., 2001 | Case-control | Norwegian Study | White men and postmenopausal women, age 60 years, n= 260 | Femoral neck BMD (DXA) | Acute stroke |
|
Adjusted for BMI, alcohol, previous MI, and medication for hypertensive |
| Mussolino et al., 2003 | Prospective | NHANES I | White and black, men and women, 4574 years, n=3402 | Phalangeal BMD (RA) | Stroke incidence | Incidence of stroke was not associated with a decrease in BMD in white men, white women, or blacks | Adjusted for age, smoking, alcohol consumption, history of diabetes, history of heart disease, education, BMI, physical activity, and blood pressure medications |
| Laroche et al., 1994 | Cross-sectional | 18 men | BMC of legs (DXA) | Symptomatic peripheral arterial disease | BMC of the more severely affected leg was lower significantly lower than BMD of the less affected leg | – | |
| Browner et al., 1993 | Prospective (1.98-years follow-up) | SOF | White, postmenopausal women, 65 years and older, n= 4024 | Calcaneal BMD (SPA) | Incident stroke |
|
Adjusted for age, follow-up time, diabetes, systolic blood pressure, alcohol, smoking, HRT use, cognitive ability, grip strength, and functional ability |
Table III -.
Effect of controlling for IL-6, TNF-α, or oxLDL on the adjusted associations of aBMD measures with incident CVD in black women, the Health, Aging, and Body Composition Study.
| BMD | N at risk (events) | Adjusted for risk factors*Hazard Ratio (95% CI) | Adjusted for risk factors + IL-6, TNF-α, or oxLDL**Hazard Ratio (95% CI) |
|---|---|---|---|
| Total Hip aBMD | |||
| IL-6 | 502 (84) | 1.39 (1.06-1.83)a | 1.39 (1.06-1.82)a |
| TNF-α | 486 (77) | 1.32 (0.99-1.76) | 1.33 (1.00-1.77) |
| oxLDL | 524 (86) | 1.32 (1.02-1.72)a | 1.35 (1.03-1.77)a |
| Femoral Neck aBMD | |||
| IL-6 | 502 (84) | 1.51 (1.14-1.99)b | 1.49 (1.13-1.96)b |
| TNF-α | 486 (77) | 1.46 (1.09-1.96)a | 1.48 (1.10-1.98)b |
| oxLDL | 524 (86) | 1.42 (1.09-1.86)b | 1.44 (1.09-1.89)b |
| Trochanter aBMD | |||
| IL-6 | 502 (84) | 1.36 (1.05-1.77)a | 1.35 (1.05-1.74)a |
| TNF-α | 486 (77) | 1.32 (1.01-1.73)a | 1.31 (1.01-1.72)a |
| oxLDL | 524 (86) | 1.32 (1.02-1.69)a | 1.34 (1.03-1.72)a |
Models in women were adjusted for age, study site, physical activity, Health ABC physical performance score, BMI, cholesterol, systolic blood pressure, glucose level, history of hypertension, and use of diabetes drugs, calcium supplements, and oral estrogen.
oxLDL models did not include cholesterol level due to the high correlation between the two measures.
p<0.05
p≤0.01
Table IV -.
Effect of controlling for IL-6, TNF-α, or oxLDL on the adjusted associations of vBMD measures with incident in white men, the Health, Aging, and Body Composition Study.
| BMD | N at risk (events) | Adjusted for risk factors*Hazard Ratio (95% CI) | Adjusted for risk factors + IL-6, TNF-α, or oxLDL**Hazard Ratio (95% CI) |
|---|---|---|---|
| Integral vBMD | |||
| IL-6 | 280 (62) | 1.37 (1.01-1.86)a | 1.38 (1.02-1.88)a |
| TNF-α | 276 (63) | 1.40 (1.04-1.89)a | 1.40 (1.04-1.89)a |
| oxLDL | 292 (66) | 1.39 (1.04-1.87)a | 1.41 (1.05-1.89)a |
| Cortical vBMD | |||
| IL-6 | 280 (62) | 1.37 (1.02-1.85)a | 1.38 (1.02-1.86)a |
| TNF-α | 276 (63) | 1.39 (1.03-1.86)a | 1.38 (1.03-1.85)a |
| oxLDL | 292 (66) | 1.39 (1.04-1.85)a | 1.41 (1.05-1.88)a |
Models in men were adjusted for: age, study site, education, physical activity, Health ABC physical performance score, BMI, HDL, LDL, systolic blood pressure, glucose level, history of hypertension, and use of diabetes drugs.
oxLDL models did not include LDL level due to the high correlation between the two measures.
p<0.05
Other studies have reported significant associations between osteoporosis and CVD in women. Results from the Multiple Outcomes of Raloxifene Evaluation (MORE) trial indicated that osteoporosis was a strong predictor of incident cardiovascular events in postmenopausal women independent of age and other traditional cardiovascular risk factors (adjusted RR = 3.9, 95% CI 2.0-7.7) (20). Osteoporosis was also associated with angiographically-determined coronary artery disease in a retrospective analysis of a population predominantly of women referred for angiography and BMD assessment (21). A report from the 30 year follow-up of the Framingham study found that metacarpal cortical area (MCA) predicts coronary heart disease in women free from CVD at baseline, with a significant trend of decreasing coronary heart disease risk with increasing MCA (RR for highest vs. lowest MCA quartile = 0.73, 95% CI 0.53-1.00, p for trend = 0.03). No association, however, was observed in men in this study (22). In SOF, low calcaneal bone mass was significantly associated with stroke incidence (RR = 1.31 per SD, 95%CI 1.03-1.65) (23). In line with these findings, low femoral neck BMD was associated with an increased odds of stroke in women, but not in men, in a Norwegian population (24).
Similar associations were also reported in men. A History of myocardial infarction was associated with low BMD in a multi-ethnic population of men in the Third National Health and Nutrition Examination Survey (NHANES III) (25). Additionally, in a study involving 18 men with asymmetrical symptomatic peripheral arterial disease, bone mineral content was shown to be significantly lower in the affected compared to the unaffected leg (26).
In contrast to the above studies, and consistent with their mortality finding, Mussolino et al. found no relationship between BMD and stroke incidence among white men, white women or blacks in NHANES I (17).
Bone mass and subclinical atherosclerosis
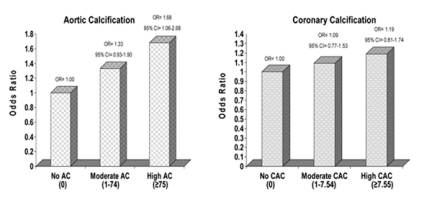
An inverse relationship between bone mass and various measures of subclinical disease, especially in women, has been reported by many studies (TableV). Cross-sectionally, vascular calcification, in both the aorta (27-33) and the coronary arteries (34, 35) was found to be negatively correlated with bone density (28-33) and directly related to vertebral and hip fractures, (28, 29) predominantly in white postmenopausal women. We observed an inverse cross-sectional association between trabecular BMD of the spine and aortic calcification in a biracial cohort of healthy middle-aged women from the Study of Women’s Health Across the Nation (SWAN). This association was not age-related, was independent of shared risk factors between BMD and calcification, and was not influenced by estradiol. Meanwhile, we noted no associations with coronary artery calcification after adjusting for age (Fig.1) (27).
Table V -.
Summary of epidemiologic studies of BMD and subclinical measures of atherosclerosis.
| Author | Design | Study | Population | BMD measurement | Subclinical atherosclerosis measure | Result | Comment |
|---|---|---|---|---|---|---|---|
| Aortic calcification | |||||||
| Farhat et al., 2007 | Cross-sectional | Study of Women’s Health Across the Nation | White and black women, 45-58 years, n= 490 | Trabecular volumetric BMD (EBCT) | Aortic calcification (AC) (EBCT) |
|
|
| Schulz et al., 2004 |
|
Study at Loma Linda University Medical Center | White postmenopausal women, 50 years and older, n= 2348 for cross-sectional and 228 for longitudinal |
|
Aortic calcification (AC) (EBCT) |
|
|
| Tanko et al., 2004 (abstract) | Cross-sectional | Prospective Epidemiological Risk Factor Study, Denmark | Postmenopausal women, aged 60-85 years, n=5409 | Hip, spine, and radius BMD (DXA) | Aortic calcification (Radiography) |
|
|
| Tanko et al., 2003 | Cross-sectional | Prospective Epidemiological Risk Factor Study, Denmark | Postmenopausal women, aged 60-85 years, n= 963 | Hip, spine, and radius BMD (DXA) | Aortic calcification (Radiography) |
|
Adjusting for intermittent claudication did not alter the association between AC and hip BMD |
| Kiel et al., 2001 | Prospective cohort (25 year follow-up) | Framingham Heart Study | White, men and women, 47-80 years, (n= 554) | Relative metacarpal cortical area (Radiogrammetry) | Aortic calcification (Radiography) |
|
Adjusted for recognized risk factors for atherosclerosis |
| Hak et al., 2000 |
|
Dutch Study | White premenopausal (n=236) and postmenopausal women (n=720), 45-64 years old | Relative metacarpal cortical area (Radiogrammetry) | Aortic calcification (Radiography) |
|
In women already postmenopausal at baseline, no association was found between progression of aortic calcification and metacarpal bone loss |
| Aoyagi et al., 2001 | Cross-sectional | Hawaii Osteoporosis Study | Japanese-American women, n= 524 | BMD at distal and proximal radius and calcaneus (SPA) | Aortic calcification (Radiography) |
|
|
| Vogt et al., 1997 | Cross-sectional | SOF | White postmenopausal women, 65 years and older, n= 2051 |
|
Aortic calcification (Radiography) |
|
|
| Banks et al., 1994 | Cross-sectional | Therapeutic RCT for prevention of postmenopausal bone loss | White early postmenopausal women, age 49-64 years, n for AC analysis= 70 |
|
Aortic calcification (defined using combination of radiography and CT) |
|
|
| Frye et al., 1992 | Cross-sectional | Study in Rochester, Minnesota | White women, 50 years and older, n= 200 |
|
Aortic calcification (Radiography) |
|
– |
| Boukhris et al., 1972 | Cross-sectional | Study in George Washington University | White and Black women (n=290) and White and Black men (n=299) | Osteoporosis of the lumbar spine (normal, moderate, severe) (Radiography) | Aortic calcification (Radiography) | Positive correlation between osteoporosis and AC in all race and gender groups | Adjusted for age only |
| Anderson et al., 1964 | Cross-sectional | Men and women attending bone clinic, n= 823 |
|
Aortic calcification (Radiography) |
|
– | |
| Coronary calcification | |||||||
| Farhat et al., 2007 | Cross-sectional | Study of Women’s Health Across the Nation | White and black women, 45-58 years, n= 490 | Trabecular volumetric BMD (EBCT) | Coronary calcification (CAC) (EBCT) |
|
– |
| Ramsey-Goldman et al., 2001 | Cross-sectional | Pilot study | 13 women with Systemic Lupus Erythematosus, mean age= 45 years, 40% menopausal, 95% white | Lumbar spine and total hip BMD (DXA) | Coronary artery calcification (EBCT) |
|
Unadjusted results |
| Barengolts et al., 1998 | Cross-sectional | Postmenopausal women, n=45 | Lumbar spine and hip BMD (DXA) | Coronary calcification (CAC) (EBCT) |
|
Unadjusted results | |
| Ankle-arm index | |||||||
| Wong et al., 2005 | Cross-sectional | Mr. and Ms Os (Hong Kong) | 3,998 Chinese men and women, 65 years and older | Lumbar spine and total hip BMD (DXA) | Ankle-arm index (<0.9) |
|
|
| van der Klift et al., 2002 | Cross-sectional | Rotterdam Study | Men and women, age 55 years and older, n=5268 | Femoral neck and spine BMD (DXA) | Ankle-arm index |
|
|
| Intima-media thickness and carotid plaque | |||||||
| Yamada et al., 2005 | Cross-sectional | Healthy Japanese population | 106 males and 154 females, mean age= 51.4 years |
|
Carotid and femoral artery IMT |
|
Adjusted for gender, age, BMI, SBP, smoking, LDL, physical functioning |
| Jørgensen et al., 2004 | Cross-sectional, Population-based | Trømso Study, Norway | Men (n=2,543) and postmenopausal women (n=2,726), aged 55-74 years | Distal and ultradistal forearm BMD (SPA) |
|
|
|
| Pennisi et al., 2004 | Case-control | Italian Study | 36 white men and postmenopausal women with peripheral atherosclerosis, 30 age and gender-matched controls |
|
|
|
– |
| Montalcini et al., 2004 | Cross-sectional | Italian Study | White postmenopausal women, aged 45-75 years, n= 157 | Calcaneal BMD (QUS) |
|
The prevalence of carotid atherosclerosis was increased in women with low BMD and osteocalcin levels above the median compared to women with low BMD and osteocalcin levels below the median (61% vs 29%, p<.05) | Women with low BMD did not have higher prevalence of atherosclerosis |
| Ramsey-Goldman et al., 2001 | Cross-sectional | Pilot study | 65 women with Systemic Lupus Erythematosus, mean age= 45 years, 40% menopausal, 95% white. | Lumbar spine and total hip BMD (DXA) | Carotid plaque index and IMT (B-mode ultrasonography) |
|
Unadjusted results |
| Uyama et al., 1997 | Cross-sectional | Japanese Study | Postmenopausal women, 67-85 years, n=30 | Lumbar spine and total BMD (DXA) | Carotid atherosclerotic plaque score (B-mode ultrasonography) |
|
Total cholesterol was also correlated with plaque score in adjusted analysis |
| Pulse wave velocity | |||||||
| Hirose et al., 2003 | Cross-sectional | Japanese study | Men and women, 21-81 years, n= 7865 | Calcaneal OSI (QUS) | Brachial-ankle pulse wave velocity |
|
All subjects had normal ankle-arm index |
| Endothelial function | |||||||
| Sanada et al., 2004 | Cross-sectional | Japanese study | Postmenopausal women, average age 53.8 years, without a history of smoking or diabetes, n= 110 | Lumbar spine BMD (DXA) | Endothelial function: forearm blood flow (FBF) at baseline, during reactive hyperemia, and after the administration of sublingual nitroglycerine | Women with osteoporosis had a lower maximal FBF response to reactive hyperemia than those with normal BMD or osteopenia | ANCOVA adjusted for age, BMI, time since menopause, and basal FBF |
Figure 1.
Adjusted odds ratios for moderate and high aortic and coronary calcification* (relative to no calcification) per 1SD decrease in vBMD**.
* Aortic calcification model: adjusted for age, race, study site, menopause status, educational level, smoking status, physical activity score, weight, height, diastolic blood pressure, LDL, and triglyceride level. Coronary artery calcification model: adjusted for age, race, study site, menopause status, alcohol drinking, physical activity score, weight, height, diastolic blood pressure, LDL, and triglyceride level.
** vBMD SD= 37.2 mg/cc
The progression of aortic calcification was also linked to volumetric trabecular BMD loss in white postmenopausal women, (28) and to metacarpal bone loss in women in the Framingham study and in a Dutch population-based longitudinal study (31, 36). Ankle-arm index was positively correlated with BMD in an elderly population of Chinese men and women (37) and in European postmenopausal women (38). In SOF, women with the highest decline in AAI were shown to have the largest magnitude of bone loss (39).
Femoral artery intima-media thickness was negatively related to calcaneal osteo-sono assessment index (OSI) in a population of Japanese men and women (40). In another small group of postmenopausal Japanese women, higher carotid plaque score was significantly associated with lower total BMD (41). Low BMD was also related to echogenic calcified carotid artery plaques in a large population of Norwegian men and postmenopausal women (42). And in a small case-control study in an Italian population of men and postmenopausal women, patients with atherosclerotic involvement of the carotid and/or femoral artery had low bone mass, and significantly lower osteocalcin and bone-specific alkaline phosphatase than controls (43). In another Italian population of postmenopausal women, the prevalence of carotid atherosclerosis was higher among women with low BMD and osteocalcin levels above the median (44).
Additionally, pulse wave velocity (PWV), a marker of early stage atherosclerosis, was inversely associated with calcaneal quantitative OSI in a large Japanese population with a median age of 50 years. This association was stronger in women than men and in pre-menopausal than postmenopausal women (45). A recent report on forearm endothelial function and spine BMD in early postmenopausal Japanese women indicated that osteoporotic women had a lower maximal forearm blood flow response to reactive hyperemia than those with normal BMD or osteopenia (46).
Other studies have failed to observe an association between osteoporosis and subclinical measures of atherosclerosis. In the Framingham Study, vascular calcification was not found to increase long-term hip fracture risk (47). In SOF, no significant association was observed between aortic calcification and bone density at the hip, spine, or calcaneus after adjusting for age; only a weak association with radial BMD was noted (48). These findings were consistent with others reported by Frye et. al. among women in Rochester, Minnesota (49), by Aoyagi et. al. in Japanese-American women (50), and by Anderson et al. in a population of British men and women (51).
Limitations of the existing epidemiologic literature
Most of the previous reports relied on white postmenopausal women and blacks have been excluded from analyses due to their reduced risk for osteoporosis and fractures (11-14, 20-24, 28-30, 32, 34, 36, 39, 44, 48, 49). Given the well-known racial differences in the burdens of CVD and osteoporosis, an investigation into the association between the two diseases in separate ethnic groups is warranted.
Additionally, a number of studies did not exclude people with baseline CVD from analyses (11, 12, 14-17, 20, 23, 24, 28, 30, 31, 36-39, 42, 44, 48, 50). Therefore, those associations might have been confounded by factors such as reduced physical activity ensuing from CVD, which in itself contributes to lower BMD. In a large number of studies, bone mass was determined using radiographic techniques, single-photon or single X-ray absorptiometry, or dual-photon absorptiometry (13-15, 17, 22, 23, 31, 33, 36, 42, 44, 45, 50, 51). Some studies have employed DXA in bone determination (11, 12, 16, 20, 21, 24-26, 29, 30, 34, 35, 37-41, 43, 46, 48); however, this technique is limited by its 2-dimensional areal assessment of BMD which does not adjust for bone size. This is especially important in studies of different ethnic and gender groups since there are well-established differences in bone size by race and gender (52, 53). DXA is also affected by the presence of extra-osseous calcium such as aortic calcification and degenerative osteoarthritic changes, which get incorporated in the region of interest and lead to a falsely increased bone density at the spine (32). This is an important drawback, particularly in the elderly who have an increased prevalence of such degenerative conditions (54). Quantitative computed tomography (QCT) allows for a three-dimensional volumetric determination of bone density, an adjustment for bone size, and an assessment of purely trabecular bone. Only a few studies have utilized QCT for BMD assessment (18, 19, 27, 28, 32).
Another limitation for the existing epidemiologic studies is that some reports did not sufficiently control for important covariates including physical activity, lipids, blood pressure, and the use of medications such as statins (11, 12, 14, 15, 17, 20, 23, 24, 26, 28, 31-39, 41, 43, 46, 48, 50, 51).
Potential mechanisms for the link between osteoporosis and cardiovascular disease
The nature of the putative link between osteoporosis and cardiovascular disease remains unclear. Traditionally, these two conditions were considered unrelated and their progression was attributed to independent age-related processes (48-50). However, recent evidence from many studies points to a link between osteoporosis and CVD that cannot be explained by age alone. While this evidence has been consistent in older populations, further support for the role of factors other than age is derived from observations in younger populations. For instance, osteoporotic fractures and cardiovascular outcomes have been shown to coexist in young women with systemic lupus erythematosus (SLE), an autoimmune systemic inflammatory disease that predominantly affects young premenopausal women. The increased risk for both conditions in this young group suggests that factors beyond age are at play in the pathogenesis of osteoporosis and CVD (35). Several hypotheses have been proposed to explain the link between the two conditions.
1. Shared risk factors
One hypothesis puts forth that the coexistence of osteoporosis and CVD is due to their shared etiological factors (such as smoking, physical activity, alcohol intake, menopause, hypertension, etc), which may simultaneously promote or inhibit atherosclerosis and bone demineralization, and could partly explain the association between the two diseases (16, 30, 55, 56). However, in many epidemiologic studies, the association between osteoporosis and CVD remained even after the adjustment of some of these risk factors.
2. Common pathophysiological mechanisms
Common pathophysiological mechanisms involving inflammatory cytokines, (43) endogenous sex hormones (16, 45), oxidized lipids (57), vitamin K deficiency (58), and vitamin D (59) were implicated in the progression of the two conditions.
Inflammatory markers and cytokines
Inflammation is known to play a central role in all stages of atherogenesis from fatty streak formation to plaque rupture (60), and there is evidence for its involvement in bone loss. Animal models suggest that osteopenia can be induced in rats by triggering a generalized inflammation through the subcutaneous administration of nonspecific irritants (such as magnesium silicate and cellulose) (61). This induced osteopenia was mainly due to inhibition of bone formation (62). Chronic inflammatory diseases such as rheumatoid arthritis, lupus, and Crohn’s disease are associated with a significant risk for secondary osteoporosis and fractures. The pathogenesis of osteoporosis in these settings is attributed to systemic inflammatory processes among other factors such as glucocorticoid therapy (63).
Inflammation is a complex process that is mediated by many cytokines including IL-1, TNF-α, and IL-6. Aging is associated with increased levels of circulating inflammatory cytokines such as IL-6 and TNF-α (64). IL-6 was shown to stimulate osteoclasts, thereby increasing the rates of bone remodeling and bone loss (65). This cytokine was also observed to act as a marker of subclinical CVD in elderly people (66) and to predict CVD mortality in relatively healthy people aged 65 years and older (67). TNF-α was also shown to stimulate bone resorption and inhibit bone formation (68). Results from the Health ABC study indicated that TNF-α and IL-6 were significantly associated with prevalent clinical and subclinical disease (69), as well as incident cardiovascular events (70). In the same cohort, elevated levels of these inflammatory cytokines were related to increased risk of fracture (71).
Other cytokines may be involved. The OPG/RANK/RANKL triad, a novel signaling pathway recognized as a key regulator of bone resorption, was also shown to play a role in vascular calcification. OPG deficient mice were found to develop early-onset osteoporosis and calcification of the aorta and renal arteries (72). In another animal study, OPG was shown to be a po-tent inhibitor of warfarin- and vitamin D-induced arterial calcification at doses known to inhibit bone resorption (73). In epidemiologic studies, low OPG levels were related to higher prevalence of osteoporosis and vertebral fractures (74). Increased osteoprotegerin levels were also associated with higher prevalence of CAD, suggesting that elevated OPG may reflect a compensatory mechanism to prevent further vascular damage (75).
Endogenous sex hormones
Estrogen deficiency has been identified as the major determinant of age-related bone loss in women and men (76, 77). Despite recent evidence from randomized, placebo-controlled trials on the adverse effects or lack of effects of postmenopausal hormone therapy on CVD outcomes (78, 79), endogenous estrogen may have protective effects on the cardiovascular system in women. Estradiol prevents endothelial dysfunction by increasing the proliferation of endothelial cells, regulating the production of endothelium-derived factors such as nitric oxide, and decreasing the expression of leukocyte adhesion molecules. It inhibits the proliferation and migration of smooth muscle cells. It is also known to improve the lipid profile (80). Estrogen receptor alpha (ESR1) was shown to have an effect on CVD susceptibility in both women and men (81). Estrogen may be involved in the pathogenesis of atherogenesis and bone loss, either directly (80, 82), or through modulation of other factors including cytokines (83) and oxidized lipids (80). The direct effect of estrogen is manifested by the expression of estrogen receptors on osteoblasts, osteoclasts (84), and vascular endothelial and smooth muscle cells (80).
Androgens also seem to have an effect on bone and vascular health. A positive correlation between testosterone levels and bone density has been observed in men and women (85, 86). Androgens were also related to cardiovascular risk factors in men (87) and perimenopausal women (88) and to aortic atherosclerosis in men (89).
Lipid metabolism and oxidized lipids
Oxidized lipids have been suggested as a potential mechanism for the paradoxical occurrence of bone loss with vascular calcification. The role of oxidized lipids in atherogenesis is well established (60, 90). In vitro, Parhami et al. have observed that lipid oxidation products including, minimally oxidized LDL, ox-PAPC (oxidized 1-palmitoyl-2-arachidonyl-snglycero-3-phosphocholine), and the isoprostane iso-PGE2, have opposite effects on the differentiation of calcifying vascular cells (CVCs) and bone cells. Oxidized lipids were found to stimulate osteoblast differentiation in CVCs as manifested by their induction of alkaline-phosphatase, a marker of osteoblastic differentiation (91), and their promotion of the formation of extensive areas of calcification in CVCs. In contrast, the same lipids were observed to inhibit osteoblast differentiation in bone by depressing the induction of alkaline phosphatase activity and reducing mineralization in pre-osteoblastic bone cells. This lead to the suggestion that the accumulation of oxidized lipids in the subendothelial space of arteries promotes arterial calcification, and its accumulation in the subendothelial space of osteons may inhibit bone mineralization (57).
A growing body of evidence suggests a negative effect of an atherogenic lipid profile on bone formation. In a cohort of postmenopausal women, plasma levels of low density lipoprotein cholesterol (LDL-C) and high density lipoprotein cholesterol (HDL-C) were negatively and positively related to BMD, respectively (92). In animal studies, an atherogenic high-fat diet was found to reduce bone formation in mice (93). The adverse effects of dyslipidemia are mediated by the resultant increase in lipid oxidation products. Increased levels of circulating lipids result in the diffusion of lipoproteins across the vascular endothelium and their accumulation inside the arterial wall and in highly vascular tissues such as the bone microenvironment. Once outside the plasma, these lipid products are subjected to oxidative modification, thus becoming biologically active molecules capable of affecting a variety of cellular processes that ultimately result in atherogenesis and bone loss (93).
In line with the lipid hypothesis, a potent class of lipid lowering drugs, the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (commonly referred to as statins), is suggested to have an effect on bone health (94, 95). Statins inhibit HMG-CoA reductase, the enzyme that catalyzes the rate-determining step of cholesterol biosynthesis, the reductive de-acylation of HMG-CoA to mevalonate. In large clinical trials, statins have demonstrated the ability to markedly reduce total cholesterol, LDL-C, and triglycerides, to increases HDL-C, and to reduce the incidence of cardiovascular events and mortality (96, 97). Recent evidence suggests that statin use is related to higher BMD (98) and reduced fracture risk (96, 97). In vitro and in animal studies, statins were found to stimulate bone formation and enhance osteoblast differentiation, by increasing the expression and production of BMP-2 by human osteoblasts (99). Like other members of the BMP family, BMP-2, is known to enhance osteoblast differentiation (100).
Another class of drugs, bisphosphonates, which inhibit bone resorption and are widely used for the treatment of osteoporosis, may have cardiovascular effects. Like statins, nitrogen-containing bisphosphonates also act on the cholesterol biosynthesis pathway, however; they target enzymes more distal in the mevalonic acid pathway than HMG Co-A reductase (95). These drugs were found to have unexpected effects on lipids in postmenopausal women with osteoporosis. Chronic intravenous therapy with neridronate was shown to decrease LDL-C and apolipoprotein B and to increase HDL-C (101).
Vitamin K deficiency
Vitamin K deficiency was suggested as a common denominator for atherosclerotic calcification and low bone mass (58). Low vitamin K intake was related to low bone density (102) and increased risk of osteoporotic fracture (103). Intake of menaquinone (vitamin K-2) was inversely associated with all-cause mortality, CHD mortality, and severe atherosclerosis in the Rotterdam study (104). Impaired vitamin K status was also linked to increased atherosclerotic calcification in postmenopausal women (105). Additionally, Jie and colleagues have observed an inverse association between markers of vitamin K status and bone mass in atherosclerotic women; whereas, no such association was found in the non-atherosclerotic group. It is speculated that the effect of vitamin K on bone demineralization and vascular calcification is mediated by a vitamin K-dependent class of proteins, gla-containing proteins, which include matrix gla protein (MGP) and osteocalcin. Gla-containing proteins are thought to be involved in calcium metabolism and in the process of calcification in bone and vascular tissues due to the calcium-binding properties of their gla residues (58). These residues are acquired post-translationally by the action of vitamin K that functions as a coenzyme for glutamate carboxylase, an enzyme that mediates the conversion of glutamate to γ-carboxyglutamate (Gla). The exact physiological role of these proteins is still not clear. However, it is hypothesized that the undercarboxylation of MGP, a mineralization inhibitor, is a risk factor for vascular calcification, and that the undercarboxylation of osteocalcin, a marker of osteoblastic activity, disrupts the normal bone remodeling process mediated by osteocalcin and results in bone loss (58).
Vitamin D metabolism
Imbalances in the calciferol endocrine system may also be involved. The role of vitamin D deficiency in the pathogenesis of osteoporosis is well-established (106). Reduced levels of vitamin D were also associated with increased incident cardiovascular disease in the Framingham Offspring Study (107).
On the other hand, excess vitamin D was shown to induce atherosclerosis and osteoporosis in humans and laboratory animals, and the use of vitamin D as a food supplement in some countries coincided with an increase in the incidence of both conditions (59). Vitamin D receptor (VDR) polymorphisms are also suggested to simultaneously contribute to the risk of both osteoporosis and CVD (108).
Hyperparathyroidism
Parathyroid hormone (PTH) is one of the main regulators of calcium homeostasis. It stimulates the release of calcium and phosphate from bones. Aging is associated with increased levels of PTH as a result of vitamin D deficiency and decreased calcium intake and absorption. Elevated PTH levels contribute to the age-related bone loss and bone fragility (109, 110). Secondary hyperparathyroidism was also linked to increased risk for fractures, cardiovascular outcomes, and vascular calcification in end-stage renal disease (110, 111).
Homocysteine
Homocysteine is a variant of the amino acid cysteine and is formed during the metabolism of methionine. Its degradation requires folic acid and vitamin B12 as cofactors. Elevated levels of homocysteine could result from genetic or nutritional factors and may lead to osteoporosis and atherosclerosis. Homo-cystinuria, a genetic disorder of cystathionine β-synthase deficiency, results in early onset osteoporosis and cardiovascular events. There is considerable evidence that elevated plasma homocysteine levels are associated with an increased risk of vascular disease. Homocysteine was reported to enhance the proliferation of vascular smooth muscle cells, inhibit the regeneration of endothelial cells, and increase lipid oxidation (112). High homocysteine levels were also associated with osteoporotic fractures (113) and reduced BMD (114). Homocysteine was observed to impair bone mineralization (115) and inhibit collagen cross-linking (116).
Other factors
Other factors implicated in the pathogenesis of atherosclerosis and bone loss include nitric oxide, endothelin-1, angiotensin converting enzyme activity, ascorbic acid, potassium, hyper-phosphatemia, oxidative stress, and the preferential differentiation of bone marrow stromal cells into smooth muscle cells over osteoblasts.
3. Common genetic factors
The osteoprotegerin, matrix-gla protein, and apolipoprotein E(ApoE) genes have been invoked in both atherogenesis and bone loss. Mice lacking the osteoprotegerin gene were found to develop early-onset osteoporosis and calcification of the aorta and renal arteries (72). Similarly, mice lacking the gene for matrix gla protein exhibited vascular calcification as well as osteopenia and fractures (117). ApoE genotype was associated with atherosclerosis in the Framingham Study and in patients with end stage renal disease (118, 119). TheApoE4 gene was also associated with reduced BMD and increased fracture risk (120, 121).
4. Causal association
Other hypotheses point to a causal association between the two conditions whereby one of them may lead to the other.
The reduced blood flow hypothesis assumes that atherosclerosis, by reducing blood flow to the lower extremities, could affect intraosseous blood circulation. This in turn alters bone metabolism in the hip and results in osteoporosis. This hypothesis is supported by a study which showed that in cases of asymmetrical peripheral arterial disease, hip bone mineral content was lower in the affected leg compared to the unaffected one (26, 122). Consistent with this finding, low ankle-arm index was associated with low BMD at the femoral neck, but not at the spine in the Rotterdam Study (38). Additionally, BMD at the hip, but not at the spine or radius, showed an inverse relation with aortic calcification - a condition thought to affect blood flow to the distal regions or reflect atherosclerosis in arteries directly responsible for blood supply to the hip (30). In line with this theory, one histological study of 100 cadavers, reported the existence of atherosclerotic changes in intraosseous arteries and arterioles of the femur (123).
Physical activity was also suggested to lie on the causal pathway between atherosclerosis and bone loss. CVD might limit physical activity and accordingly contribute to bone loss (23). It is also hypothesized that as a result of the progressive bone loss leading to osteoporosis, calcium and phosphate salts get redirected from the bone matrix to the arterial wall (33, 34, 48, 124, 125).
Future research
Additional longitudinal studies are needed to confirm the association between osteoporosis and CVD. Furthermore, racial differences in this association deserve further investigation. Examination of the relation between bone loss and the progression of vascular calcification is certainly warranted. A subclinical assessment of CVD may allow for osteoporosis risk stratification and the early identification of subjects at high risk for developing the condition, and vice versa. Another key avenue for future research is the elucidation of the common mechanisms underlying the link between osteoporosis and CVD. An understanding of these mechanisms will set the stage for the potential use of common preventive and therapeutic interventions targeted at both conditions.
Conclusion
CVD and osteoporosis are major causes of morbidity, mortality, and disability. Both diseases increase with aging. Traditionally, these two conditions were considered unrelated and their coexistence was attributed to independent age-related processes. Recently, an increasing body of biological and epidemiological evidence has provided support for a link between the two conditions beyond age and shared risk factors. It is suggested that common molecular, cellular, and biochemical processes are implicated in their pathogenesis. New paradigms for treatment and prevention of both CVD and osteoporosis may emerge from investigating the link between the two conditions and elucidating the mechanisms involved in their progression. An understanding of the biological linkages may set the stage for dual-purpose preventive and therapeutic interventions aimed at reducing bone loss and the progression of atherosclerosis.
Footnotes
Relative Risk (RR) was used to refer to both Risk Ratios and Hazard Ratios.
References
- 1.Doherty TM, Detrano RC. Coronary arterial calcification as an active process: a new perspective on an old problem. Calcif Tissue Int. 1994;54:224–230. doi: 10.1007/BF00301683. [DOI] [PubMed] [Google Scholar]
- 2.Vattikuti R, Towler DA. Osteogenic regulation of vascular calcification: an early perspective. Am J Physiol Endocrinol Metab. 2004;286:E686–E696. doi: 10.1152/ajpendo.00552.2003. [DOI] [PubMed] [Google Scholar]
- 3.Bostrom K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800–1809. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmid K, McSharry WO, Pameijer CH, Binette JP. Chemical and physicochemical studies on the mineral deposits of the human atherosclerotic aorta. Atherosclerosis. 1980;37:199–210. doi: 10.1016/0021-9150(80)90005-2. [DOI] [PubMed] [Google Scholar]
- 5.Hsu HHT, Camacho NP. Isolation of calcifiable vesicles from human atherosclerotic aorta. Atherosclerosis. 1999;143:353–362. doi: 10.1016/s0021-9150(98)00322-0. [DOI] [PubMed] [Google Scholar]
- 6.Shanahan CM, Cary NR, Metcalfe JC, Weissberg PL. High expression of genes for calcification-regulating proteins in human atherosclerotic plaques. J Clin Invest. 1994;93:2393–2402. doi: 10.1172/JCI117246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giachelli CM, Bae N, Almeida M, Denhardt DT, Alpers CE, Schwartz SM. Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. J Clin Invest. 1993;92:1686–1696. doi: 10.1172/JCI116755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, Tordoir JH, Spronk HM, Vermeer C, Daemen MJ. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- 9.Watson KE, Bostrom K, Ravindranath R, Lam T, Norton B, Demer LL. TGF-beta 1 and 25-hydroxycholesterol stimulate osteoblast-like vascular cells to calcify. J Clin Invest. 1994;93:2106–2113. doi: 10.1172/JCI117205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doherty TM, Fitzpatrick LA, Inoue D, Qiao J-H, Fishbein MC, Detrano RC, Shah PK, Rajavashisth TB. Molecular, Endocrine, and Genetic Mechanisms of Arterial Calcification. Endocr Rev. 2004;25:629–672. doi: 10.1210/er.2003-0015. [DOI] [PubMed] [Google Scholar]
- 11.Bauer DC, Palermo D, Black D, Cauley JA. Quantitative ultrasound and mortality: a prospective study. Osteoporos Int. 2002;13:606–612. doi: 10.1007/s001980200081. [DOI] [PubMed] [Google Scholar]
- 12.Kado DM, Browner WS, Blackwell T, Gore R, Cummings SR. Rate of bone loss is associated with mortality in older women: a prospective study. J Bone Miner Res. 2000;15:1974–1980. doi: 10.1359/jbmr.2000.15.10.1974. [DOI] [PubMed] [Google Scholar]
- 13.von der Recke P, Hansen MA, Hassager C. The association between low bone mass at the menopause and cardiovascular mortality. Am J Med. 1999;106:273–278. doi: 10.1016/s0002-9343(99)00028-5. [DOI] [PubMed] [Google Scholar]
- 14.Browner WS, Seeley DG, Vogt TM, Cummings SR. Non-trauma mortality in elderly women with low bone mineral density. Study of Osteoporotic Fractures Research Group. Lancet. 1991;338:355–358. doi: 10.1016/0140-6736(91)90489-c. [DOI] [PubMed] [Google Scholar]
- 15.Mussolino ME, Madans JH, Gillum RF. Bone mineral density and mortality in women and men: the NHANES I epidemiologic follow-up study. Ann Epidemiol. 2003;13:692–697. doi: 10.1016/s1047-2797(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 16.Trivedi DP, Khaw KT. Bone mineral density at the hip predicts mortality in elderly men. Osteoporos Int. 2001;12:259–265. doi: 10.1007/s001980170114. [DOI] [PubMed] [Google Scholar]
- 17.Mussolino ME, Madans JH, Gillum RF. Bone mineral density and stroke. Stroke. 2003;34:e20–22. doi: 10.1161/01.STR.0000065826.23815.A5. [DOI] [PubMed] [Google Scholar]
- 18.Farhat GN, Strotmeyer ES, Newman AB, Sutton-Tyrrell K, Bauer DC, Harris T, Johnson KC, Taaffe DR, Cauley JA. Volumetric and areal bone mineral density measures are associated with cardiovascular disease in older men and women: the health, aging, and body composition study. Calcif Tissue Int. 2006;79:102–111. doi: 10.1007/s00223-006-0052-0. [DOI] [PubMed] [Google Scholar]
- 19.Farhat GN, Newman AB, Sutton-Tyrrell K, Matthews KA, Boudreau R, Schwartz AV, Harris T, Tylavsky F, Visser M, Cauley JA. The association of bone mineral density measures with incident cardiovascular disease in older adults. Osteoporos Int. 2007;18:999–1008. doi: 10.1007/s00198-007-0338-8. [DOI] [PubMed] [Google Scholar]
- 20.Tanko L, Christiansen C, Cox DA, Geiger MJ, McNabb MA, Cummings SR. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J Bone Miner Res. 2005;20:1912–1920. doi: 10.1359/JBMR.050711. [DOI] [PubMed] [Google Scholar]
- 21.Marcovitz PA, Tran HH, Franklin BA, O'Neill WW, Yerkey M, Boura J, Kleerekoper M, Dickinson CZ. Usefulness of bone mineral density to predict significant coronary artery disease. Am J Cardiol. 2005;96:1059–1063. doi: 10.1016/j.amjcard.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 22.Samelson EJ, Kiel DP, Broe KE, Zhang Y, Cupples LA, Hannan MT, Wilson PW, Levy D, Williams SA, Vaccarino V. Metacarpal cortical area and risk of coronary heart disease: the Framingham Study. Am J Epidemiol. 2004;159:589–595. doi: 10.1093/aje/kwh080. [DOI] [PubMed] [Google Scholar]
- 23.Browner WS, Pressman AR, Nevitt MC, Cauley JA, Cummings SR. Association between low bone density and stroke in elderly women. The study of osteoporotic fractures. Stroke. 1993;24:940–946. doi: 10.1161/01.str.24.7.940. [DOI] [PubMed] [Google Scholar]
- 24.Jorgensen L, Engstad T, Jacobsen BK. Bone mineral density in acute stroke patients: low bone mineral density may predict first stroke in women. Stroke. 2001;32:47–51. doi: 10.1161/01.str.32.1.47. [DOI] [PubMed] [Google Scholar]
- 25.Magnus JH, Broussard DL. Relationship between bone mineral density and myocardial infarction in US adults. Osteoporos Int. 2005;16:2053–2062. doi: 10.1007/s00198-005-1999-9. [DOI] [PubMed] [Google Scholar]
- 26.Laroche M, Pouilles JM, Ribot C, Bendayan P, Bernard J, Boccalon H, Mazieres B. Comparison of the bone mineral content of the lower limbs in men with ischaemic atherosclerotic disease. Clin Rheumatol. 1994;13:611–614. doi: 10.1007/BF02243003. [DOI] [PubMed] [Google Scholar]
- 27.Farhat GN, Cauley JA, Matthews KA, Newman AB, Johnston J, Mackey R, Edmundowicz D, Sutton-Tyrrell K. Volumetric BMD and vascular calcification in middle-aged women: the Study of Women's Health Across the Nation. J Bone Miner Res. 2006;21:1839–1846. doi: 10.1359/jbmr.060903. [DOI] [PubMed] [Google Scholar]
- 28.Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V. Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab. 2004;89:4246–4253. doi: 10.1210/jc.2003-030964. [DOI] [PubMed] [Google Scholar]
- 29.Tanko LB, Bagger YZ, Alexandersen P, Christiansen C. Osteoporotic and Cardiovascular Disease are Highly Correlated Co-morbidities in Elderly Women. JBMR. 2004;19(Suppl 1):S91. [Google Scholar]
- 30.Tanko LB, Bagger YZ, Christiansen C. Low bone mineral density in the hip as a marker of advanced atherosclerosis in elderly women. Calcif Tissue Int. 2003;73:15–20. doi: 10.1007/s00223-002-2070-x. [DOI] [PubMed] [Google Scholar]
- 31.Hak AE, Pols HA, van Hemert AM, Hofman A, Witteman JC. Progression of aortic calcification is associated with metacarpal bone loss during menopause: a population-based longitudinal study. Arterioscler Thromb Vasc Biol. 2000;20:1926–1931. doi: 10.1161/01.atv.20.8.1926. [DOI] [PubMed] [Google Scholar]
- 32.Banks LM, Lees B, Macsweeney JE, Stevenson JC. Effect of degenerative spinal and aortic calcification on bone density measurements in postmenopausal women women: links between osteoporosis and cardiovascular disease? Eur J Clin Invest. 1994;24:813–817. doi: 10.1111/j.1365-2362.1994.tb02024.x. [DOI] [PubMed] [Google Scholar]
- 33.Boukhris R, Becker KL. Calcification of the Aorta and Osteoporosis. JAMA. 1972;219:1307–1311. [PubMed] [Google Scholar]
- 34.Barengolts EI, Berman M, Kukreja SC, Kouznetsova T, Lin C, Chomka EV. Osteoporosis and coronary atherosclerosis in asymptomatic postmenopausal women. Calcif Tissue Int. 1998;62:209–213. doi: 10.1007/s002239900419. [DOI] [PubMed] [Google Scholar]
- 35.Ramsey-Goldman R, Manzi S. Association of osteoporosis with cardiovascular disaese in women with systemic lupus erythematosus. Arthritis and Rheumatism. 2001;44:2338–2341. doi: 10.1002/1529-0131(200110)44:10<2338::aid-art396>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 36.Kiel DP, Kauppila LI, Cupples LA, Hannan MT, O'Donnell CJ, Wilson PW. Bone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham Heart Study. Calcif Tissue Int. 2001;68:271–276. doi: 10.1007/BF02390833. [DOI] [PubMed] [Google Scholar]
- 37.Wong SYS, Kwok T, Woo J, Lynn H, Griffith JF, Leung J, Tang YYN, Leung PC. Bone mineral density and the risk of peripheral arterial disease in men and women: results from the Mr. and Ms. Os, Hong Kong. Osteoporos Int. 2005;16:1933–1938. doi: 10.1007/s00198-005-1968-3. [DOI] [PubMed] [Google Scholar]
- 38.van der Klift M, Pols HA, Hak AE, Witteman JC, Hofman A, de Laet CE. Bone mineral density and the risk of peripheral arterial disease: the Rotterdam Study. Calcif Tissue Int. 2002;70:443–449. doi: 10.1007/s00223-001-2076-9. [DOI] [PubMed] [Google Scholar]
- 39.Vogt MT, Cauley JA, Kuller LH, Nevitt MC. Bone mineral density and blood flow to the lower extremities: the study of osteoporotic fractures. J Bone Miner Res. 1997;12:283–289. doi: 10.1359/jbmr.1997.12.2.283. [DOI] [PubMed] [Google Scholar]
- 40.Yamada S, IInaba M, Goto H, Nagata A, Ueda M, Emoto M, Shoji T, Nishizawa Y. Significance of intima-media thickness in femoral artery in the determination of calcaneus osteo-sono index but not of lumbar spine bone mass in healthy Japanese people. Osteoporos Int. 2005;16:64–70. doi: 10.1007/s00198-004-1642-1. [DOI] [PubMed] [Google Scholar]
- 41.Uyama O, Yoshimoto Y, Yamamoto Y, Kawai A. Bone changes and carotid atherosclerosis in postmenopausal women. Stroke. 1997;28:1730–1732. doi: 10.1161/01.str.28.9.1730. [DOI] [PubMed] [Google Scholar]
- 42.Jorgensen L, Joakimsen O, Rosvold Berntsen GK, Heuch I, Jacobsen BK. Low bone mineral density is related to echogenic carotid artery plaques: a population-based study. Am J Epidemiol. 2004;160:549–556. doi: 10.1093/aje/kwh252. [DOI] [PubMed] [Google Scholar]
- 43.Pennisi P, Signorelli SS, Riccobene S, Celotta G, Di Pino L, La Malfa T, Fiore CE. Low bone density and abnormal bone turnover in patients with atherosclerosis of peripheral vessels. Osteoporos Int. 2004;15:389–395. doi: 10.1007/s00198-003-1550-9. [DOI] [PubMed] [Google Scholar]
- 44.Montalcini T, Emanuele V, Ceravolo R, Gorgone G, Sesti G, Perticone F, Pujia A. Relation of low bone mineral density and carotid atherosclerosis in postmenopausal women. Am J Cardiol. 2004;94:266–269. doi: 10.1016/j.amjcard.2004.03.083. [DOI] [PubMed] [Google Scholar]
- 45.Hirose K, Tomiyama H, Okazaki R, Arai T, Koji Y, Zaydun G, Hori S, Yamashina A. Increased pulse wave velocity associated with reduced calcaneal quantitative osteo-sono index: possible relationship between atherosclerosis and osteopenia. J Clin Endocrinol Metab. 2003;88:2573–2578. doi: 10.1210/jc.2002-021511. [DOI] [PubMed] [Google Scholar]
- 46.Sanada M, Taguchi A, Higashi Y, Tsuda M, Kodama I, Yoshizumi M, Ohama K. Forearm endothelial function and bone mineral loss in postmenopausal women. Atherosclerosis. 2004;176:387–392. doi: 10.1016/j.atherosclerosis.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 47.Samelson EJ, Cupples LA, Broe KE, Hannan MT, O'Donnell CJ, Kiel DP. Vascular calcification in middle age and long-term risk of hip fracture: the Framingham Study. J Bone Miner Res. 2007;22:1449–1454. doi: 10.1359/jbmr.070519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vogt MT, San Valentin R, Forrest KY, Nevitt MC, Cauley JA. Bone mineral density and aortic calcification: the Study of Osteoporotic Fractures. J Am Geriatr Soc. 1997;45:140–145. doi: 10.1111/j.1532-5415.1997.tb04498.x. [DOI] [PubMed] [Google Scholar]
- 49.Frye MA, Melton LJ, Melton LJ 3rd, Bryant SC, Fitzpatrick LA, Wahner HW, Schwartz RS, Riggs BL. Osteoporosis and calcification of the aorta. Bone Miner. 1992;19:185–194. doi: 10.1016/0169-6009(92)90925-4. [DOI] [PubMed] [Google Scholar]
- 50.Aoyagi K, Ross PD, Orloff J, Davis JW, Katagiri H, Wasnich RD. Low bone density is not associated with aortic calcification. Calcif Tissue Int. 2001;69:20–24. doi: 10.1007/s002230020003. [DOI] [PubMed] [Google Scholar]
- 51.Anderson JB, Barnett E, Nordin BEC. The relation between osteoporosis and aortic calcification. Brit J Radiol. 1964;37:910–912. doi: 10.1259/0007-1285-37-444-910. [DOI] [PubMed] [Google Scholar]
- 52.Looker AC, Beck TJ, Orwoll ES. Does body size account for gender differences in femur bone density and geometry? J Bone Miner Res. 2001;16:1291–1299. doi: 10.1359/jbmr.2001.16.7.1291. [DOI] [PubMed] [Google Scholar]
- 53.Seeman E. Editorial: Growth in bone mass and size- Are racial and gender differences in bone mineral density more apprent than real? J Clin Endocrinol Metab. 1998;83:1414–1419. doi: 10.1210/jcem.83.5.4844. [DOI] [PubMed] [Google Scholar]
- 54.Rand T, Seidl G, Kainberger F, Resch A, Hittmair K, Schneider B, Glüer C, Imhof H. Impact of Spinal Degenerative Changes on the Evaluation of Bone Mineral Density with Dual Energy X-Ray Absorptiometry (DXA) Calcif Tissue Int. 1997;60:430–433. doi: 10.1007/s002239900258. [DOI] [PubMed] [Google Scholar]
- 55.McFarlane SI, Muniyappa R, Shin JJ, Bahtiyar G, Sowers JR. Osteoporosis and cardiovascular disease: brittle bones and boned arteries, is there a link? Endocrine. 2004;23:1–10. doi: 10.1385/ENDO:23:1:01. [DOI] [PubMed] [Google Scholar]
- 56.Alagiakrishnan K, Juby A, Hanley D, Tymchak W, Sclater A. Role of vascular factors in osteoporosis. J Gerontol A Biol Sci Med Sci. 2003;58:362–366. doi: 10.1093/gerona/58.4.m362. [DOI] [PubMed] [Google Scholar]
- 57.Parhami F, Morrow AD, Balucan J, Leitinger N, Watson AD, Tintut Y, Berliner JA, Demer LL. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997;17:680–687. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- 58.Jie KG, Bots ML, Vermeer C, Witteman JC, Grobbee DE. Vitamin K status and bone mass in women with and without aortic atherosclerosis: a population-based study. Calcif Tissue Int. 1996;59:352–356. doi: 10.1007/s002239900139. [DOI] [PubMed] [Google Scholar]
- 59.Moon J, Bandy B, Davison AJ. Hypothesis: etiology of atherosclerosis and osteoporosis: are imbalances in the calciferol endocrine system implicated? J Am Coll Nutr. 1992;11:567–583. doi: 10.1080/07315724.1992.10718263. [DOI] [PubMed] [Google Scholar]
- 60.Hansson GK. Inflammation, Atherosclerosis, and Coronary Artery Disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 61.Minne HW, Pfeilschifter J, Scharla S, Mutschelknauss S, Schwarz A, Krempien B, Ziegler R. Inflammation-mediated osteopenia in the rat: a new animal model for pathological loss of bone mass. Endocrinology. 1984;115:50–54. doi: 10.1210/endo-115-1-50. [DOI] [PubMed] [Google Scholar]
- 62.Lempert UG, Minne HW, Fleisch H, Muhlbauer RC, Scharla SH, Ziegler R. Inflammation-mediated osteopenia (IMO): no change in bone resorption during its development. Calcif Tissue Int. 1991;48:291–292. doi: 10.1007/BF02556383. [DOI] [PubMed] [Google Scholar]
- 63.Scharla SH, Schacht E, Lempert UG. Alfacalcidol versus plain vitamin D in inflammation induced bone loss. J Rheumatol Suppl. 2005;76:26–32. [PubMed] [Google Scholar]
- 64.Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39:687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 65.Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128:127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- 66.Jenny NS, Tracy RP, Ogg MS, Luong le A, Kuller LH, Arnold AM, Sharrett AR, Humphries SE. In the elderly, interleukin-6 plasma levels and the -174G>C polymorphism are associated with the development of cardiovascular disease. Arterioscler Thromb Vasc Biol. 2002;22:2066–2071. doi: 10.1161/01.atv.0000040224.49362.60. [DOI] [PubMed] [Google Scholar]
- 67.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH Jr., Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 68.Bertolini DR, Nedwin GE, Bringman TS, Smith DD, Mundy GR. Stimulation of bone resorption and inhibition of bone formationin vitro by human tumor necrosis factors. Nature. 1986;319:516–518. doi: 10.1038/319516a0. [DOI] [PubMed] [Google Scholar]
- 69.Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Tracy RP, Rubin SM, Harris TB, Pahor M. Inflammatory markers and cardiovascular disease (The Health, Aging and Body Composition (Health ABC) Study) Am J Cardiol. 2003;92:522–528. doi: 10.1016/s0002-9149(03)00718-5. [DOI] [PubMed] [Google Scholar]
- 70.Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Rubin SM, Ding J, Simonsick EM, Harris TB, Pahor M. Inflammatory Markers and Onset of Cardiovascular Events. Results From the Health ABC Study. Circulation. 2003;108:2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 71.Cauley JA, Danielson ME, Boudreau R, Forrest KYZ, Zmuda JM, Pahor M, Tylavsky F, Cummings SR, Harris T, Newman AB. Inflammatory markers and incident fracture risk in older men and women: The Health Aging and Body Composition Study. J Bone Miner Res. 2007;22:2007. doi: 10.1359/jbmr.070409. [DOI] [PubMed] [Google Scholar]
- 72.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Price PA, June HH, Buckley JR, Williamson MK. Osteoprotegerin inhibits artery calcification induced by warfarin and by vitamin D. Arterioscler Thromb Vasc Biol. 2001;21:1610–1616. doi: 10.1161/hq1001.097102. [DOI] [PubMed] [Google Scholar]
- 74.Mezquita-Raya P, de la Higuera M, Garcia DF, Alonso G, Ruiz-Requena ME, de Dios Luna J, Escobar-Jimenez F, Munoz-Torres M. The contribution of serum osteoprotegerin to bone mass and vertebral fractures in postmenopausal women. Osteoporos Int. 2005;16:1368–1374. doi: 10.1007/s00198-005-1844-1. [DOI] [PubMed] [Google Scholar]
- 75.Schoppet M, Sattler AM, Schaefer JR, Herzum M, Maisch B, Hofbauer LC. Increased osteoprotegerin serum levels in men with coronary artery disease. J Clin Endocrinol Metab. 2003;88:1024–1028. doi: 10.1210/jc.2002-020775. [DOI] [PubMed] [Google Scholar]
- 76.Riggs BL, Khosla S, Melton LJ. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 77.Khosla S, Melton LJ, Riggs BL. Clinical review 144: Estrogen and the male skeleton. J Clin Endocrinol Metab. 2002;87:1443–1450. doi: 10.1210/jcem.87.4.8417. [DOI] [PubMed] [Google Scholar]
- 78.Writing Group for the Women's Health Initiative Investigators. Risks and Benefits of Estrogen Plus Progestin in Healthy Postmenopausal Women: Principal Results From the Women’s Health Initiative Randomized Controlled Trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 79.Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, Hsia J, Hulley S, Herd A, Khan S, Newby LK, Waters D, Vittinghoff E, Wenger N. Cardiovascular Disease Outcomes During 6.8 Years of Hormone Therapy: Heart and Estrogen/Progestin Replacement Study Follow-up (HERS II) JAMA. 2002;288:49–57. doi: 10.1001/jama.288.1.49. for the HERS Research Group. [DOI] [PubMed] [Google Scholar]
- 80.Mendelsohn ME. Protective effects of estrogen on the cardiovascular system. Am J Cardiol. 2002;20:12E–17E. doi: 10.1016/s0002-9149(02)02405-0. [DOI] [PubMed] [Google Scholar]
- 81.Shearman AM, Cupples LA, Demissie S, Peter I, Schmid CH, Karas RH, Mendelsohn ME, Housman DE, Levy D. Association between estrogen receptor alpha gene variation and cardiovascular disease. JAMA. 2003;290:2263–2270. doi: 10.1001/jama.290.17.2263. [DOI] [PubMed] [Google Scholar]
- 82.Khosla S, Atkinson EJ, Melton LJ, Riggs BL. Effects of age and estrogen status on serum parathyroid hormone levels and biochemical markers of bone turnover in women: a population-based study. J Clin Endocrinol Metab. 1997;82:1522–1527. doi: 10.1210/jcem.82.5.3946. [DOI] [PubMed] [Google Scholar]
- 83.Jilka RL, Hangoc G, Girasole G, Passeri G, Williams DC, Abrams JS, Boyce B, Broxmeyer H, Manolagas SC. Increased Osteoclast Development After Estrogen Loss: Mediation by Interleukin-6. Science. 1992:257. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- 84.Monroe DG, Spelsberg TC. Gonadal Steroids and Receptors. In: Favus MJ (ed) Primer on the Metabolic Bone Diseases and Disroders of mineral Metabolism. American Society for Bone and Mineral Research. 2003:32–38. Washington, DC. [Google Scholar]
- 85.Khosla S, Melton LJ, Riggs BL. Dietary calcium, sex hormones, and bone mineral density in men. J Clin Endocrinol Metab. 2002;87:1443–1450. doi: 10.1210/jcem.87.4.8417. [DOI] [PubMed] [Google Scholar]
- 86.Wild RA, Buchanan JR, Myers C, Demers LM. Declining adrenal androgens: an association with bone loss in aging women. Proc Soc Exp Biol Med. 1987;186:355–360. doi: 10.3181/00379727-186-42625. [DOI] [PubMed] [Google Scholar]
- 87.Khaw KT, Barrett-Connor E. Endogenous sex hormones, high density lipoprotein cholesterol, and other lipoprotein fractions in men. Arterioscler Thromb Vasc Biol. 1991;11:489–494. doi: 10.1161/01.atv.11.3.489. [DOI] [PubMed] [Google Scholar]
- 88.Sutton-Tyrrell K, Wildman RP, Matthews KA, Chae C, Lasley BL, Brockwell S, Pasternak RC, Lloyd-Jones D, Sowers MF, Torrens JI, Investigators S. Sex hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN) Circulation. 2005;111:1242–1249. doi: 10.1161/01.CIR.0000157697.54255.CE. [DOI] [PubMed] [Google Scholar]
- 89.Hak AE, Witteman JC, de Jong FH, Geerlings MI, Hofman A, Pols HA. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab. 2002;87:3632–3639. doi: 10.1210/jcem.87.8.8762. [DOI] [PubMed] [Google Scholar]
- 90.Stocker R, Keaney JF. Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 91.Stein GS, Lian JB. Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocr Rev. 1993;14:424–442. doi: 10.1210/edrv-14-4-424. [DOI] [PubMed] [Google Scholar]
- 92.Yamaguchi T, Sugimoto T, Yano S, Yamauchi M, Sowa H, Chen Q, Chihara K. Plasma lipids and osteoporosis in postmenopausal women. Endocr J. 2002;49:211–217. doi: 10.1507/endocrj.49.211. [DOI] [PubMed] [Google Scholar]
- 93.Parhami F, Tintut Y, Beamer WG, Gharavi N, Goodman W, Demer LL. Atherogenic high-fat diet reduces bone mineralization in mice. JBMR. 2001;16:182–188. doi: 10.1359/jbmr.2001.16.1.182. [DOI] [PubMed] [Google Scholar]
- 94.Whitney C, Warburton DER, Frohlich J, Chan SY, McKay H, Khan K. Are Cardiovascular Disease and Osteoporosis Directly Linked? Sports Med. 2004;34:779–807. doi: 10.2165/00007256-200434120-00001. [DOI] [PubMed] [Google Scholar]
- 95.Burnett JR, Vasikaran SD. Cardiovascular disease and osteoporosis: is there a link between lipids and bone? Ann Clin Biochem. 2002;39:203–210. doi: 10.1258/0004563021902134. [DOI] [PubMed] [Google Scholar]
- 96.Bauer DC, Mundy GR, Jamal SA, Black DM, Cauley JA, Ensrud KE, van der Klift M, Pols HA. Use of Statins and Fracture. Results of 4 Prospective Studies and Cumulative Meta-analysis of Observational Studies and Controlled Trials. Arch Intern Med. 2004;164:146–152. doi: 10.1001/archinte.164.2.146. [DOI] [PubMed] [Google Scholar]
- 97.Pasco JA, Kotowicz MA, Henry MJ, Sanders KM, Nicholson GC. Statin use, bone mineral density, and fracture risk. Arch Intern Med. 2002:162. doi: 10.1001/archinte.162.5.537. [DOI] [PubMed] [Google Scholar]
- 98.Solomon DH, Finkelstein JS, Wang P, Avorn J. Statin lipid-lowering drugs and bone mineral density. Pharmacoepidemiol Drug Saf. 2005;14:219–226. doi: 10.1002/pds.984. [DOI] [PubMed] [Google Scholar]
- 99.Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, Boyce B, Zhao M, Gutierrez G. Stimulation of Bone Formation in Vitro and in Rodents by Statins. Science. 1999;286:1946–1949. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 100.Chen D, Harris MA, Rossini G, Dunstan CR, Dallas SL, Feng JQ, Mundy GR, Harris SE. Bone morphogenetic protein 2 (BMP-2) enhances BMP-3, BMP-4, and bone cell differentiation marker gene expression during the induction of mineralized bone matrix formation in cultures of fetal rat calvarial osteoblasts. Calcif Tissue Int. 1997;60:283–290. doi: 10.1007/s002239900230. [DOI] [PubMed] [Google Scholar]
- 101.Adami S, Braga V, Guidi G, Gatti D, Gerardi D, Fracassi E. Chronic intravenous aminobisphosphonate therapy increases high-density lipoprotein cholesterol and decreases low-density lipoprotein cholesterol. J Bone Miner Res. 2000;15:599–604. doi: 10.1359/jbmr.2000.15.3.599. [DOI] [PubMed] [Google Scholar]
- 102.Booth SL, Broe KE, Gagnon DR, Tucker KL, Hannan MT, McLean RR, Dawson-Hughes B, Wilson PW, Cupples LA, Kiel DP. Vitamin K intake and bone mineral density in women and men. Am J Clin Nutr. 2003;77:512–516. doi: 10.1093/ajcn/77.2.512. [DOI] [PubMed] [Google Scholar]
- 103.Booth SL, Tucker KL, Chen H, Hannan MT, Gagnon DR, Cupples LA, Wilson PW, Ordovas J, Schaefer EJ, Dawson-Hughes B, Kiel DP. Dietary vitamin K intakes are associated with hip fracture but not with bone mineral density in elderly men and women. Am J Clin Nutr. 2000;71:1201–1208. doi: 10.1093/ajcn/71.5.1201. [DOI] [PubMed] [Google Scholar]
- 104.Geleijnse JM, Vermeer C, Grobbee DE, Schurgers LJ, Knapen MHJ, van der Meer IM, Hofman A, Witteman JC. Dietary Intake of Menaquinone Is Associated with a Reduced Risk of Coronary Heart Disease: The Rotterdam Study. J Nutr. 2004;134:3100–3105. doi: 10.1093/jn/134.11.3100. [DOI] [PubMed] [Google Scholar]
- 105.Jie KS, Bots ML, Vermeer C, Witteman JC, Grobbee DE. Vitamin K intake and osteocalcin levels in women with and without aortic atherosclerosis: a population-based study. Atherosclerosis. 1995;116:117–123. doi: 10.1016/0021-9150(95)05537-7. [DOI] [PubMed] [Google Scholar]
- 106.Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115:3318–3325. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D'Agostino RB, Wolf M, Vasan RS. Vitamin d deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kammerer CM, Dualan AA, Samollow PB, Perisse ARS, Bauer RL, MacCluer JW, O'Leary DH, Mitchell BD. Bone mineral density, carotid artery intima media thickness, and the vitamin D receptor BsmI polymorphism in Mexican American women. Calcif Tissue Int. 2004;75:292–298. doi: 10.1007/s00223-004-0215-9. [DOI] [PubMed] [Google Scholar]
- 109. Eastell R. Pathogenesis of postmenopausal osteoporosis In: Favus MJ (ed) Primer on the Metabolic Bone Diseases and Disroders of mineral Metabolism American Society for Bone and Mineral Research; Washington, DC: 2003. 314 316 [Google Scholar]
- 110.de Francisco AL. Secondary hyperparathyroidism: review of the disease and its treatment. Clin Ther. 2004;26:1976–1993. doi: 10.1016/j.clinthera.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 111.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Cher-tow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 112.Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A Quantitative Assessment of Plasma Homocysteine as a Risk Factor for Vascular Disease: Probable Benefits of Increasing Folic Acid Intakes. JAMA. 1995;274:1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 113.McLean RR, Jacques PF, Selhub J, Tucker KL, Samelson EJ, Broe KE, Hannan MT, Cupples LA, Kiel DP. Homocysteine as a Predictive Factor for Hip Fracture in Older Persons. N Engl J Med. 2004;350:2042–2049. doi: 10.1056/NEJMoa032739. [DOI] [PubMed] [Google Scholar]
- 114.Gjesdal CG, Vollset SE, Ueland PM, Refsum H, Drevon CA, Gjessing HK, Tell GS. Plasma total homocysteine level and bone mineral density: the Hordaland Homocysteine Study. Arch Intern Med. 2006;166:88–94. doi: 10.1001/archinte.166.1.88. [DOI] [PubMed] [Google Scholar]
- 115.Khan M, Yamauchi M, Srisawasdi S, Stiner D, Doty S, Paschalis EP, Boskey AL. Homocysteine decreases chondrocyte-mediated matrix mineralization in differentiating chick limb-bud mesenchymal cell micro-mass cultures. Bone. 2001;28:387–398. doi: 10.1016/s8756-3282(01)00409-4. [DOI] [PubMed] [Google Scholar]
- 116.Lubec B, Fang-Kircher S, Lubec T, Blom HJ, Boers GH. Evidence for McKusick's hypothesis of deficient collagen cross-linking in patients with homocystinuria. Biochim Biophys Acta. 1996;1315:159–162. doi: 10.1016/0925-4439(95)00119-0. [DOI] [PubMed] [Google Scholar]
- 117.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 118.Elosua R, Ordovas JM, Cupples LA, Fox CS, Polak JF, Wolf PA, D'Agostin RA, O’Donnell CJ. Association of APOE genotype with carotid atherosclerosis in men and women: the Framingham Heart Study. J Lipid Res. 2004;45:1868–1875. doi: 10.1194/jlr.M400114-JLR200. [DOI] [PubMed] [Google Scholar]
- 119.Olmer M, Renucci JE, Planells R, Bouchouareb D, Purgus R. Preliminary evidence for a role of apolipoprotein E alleles in identifying haemodialysis patients at high vascular risk. Nephrol Dial Transplant. 1997;12:691–693. doi: 10.1093/ndt/12.4.691. [DOI] [PubMed] [Google Scholar]
- 120.Shiraki M, Shiraki Y, Aoki C, Hosoi T, Inoue S, Kaneki M, Ouchi Y. Association of bone mineral density with apolipoprotein E phenotype. J Bone Miner Res. 1997;12:1438–1445. doi: 10.1359/jbmr.1997.12.9.1438. [DOI] [PubMed] [Google Scholar]
- 121.Cauley JA, Zmuda JM, Yaffe K, Kuller LH, Ferrell RE, Wisniewski SR, Cummings SR. Apolipoprotein E polymorphism: A new genetic marker of hip fracture risk--The Study of Osteoporotic Fractures. J Bone Miner Res. 1999;14:1175–1181. doi: 10.1359/jbmr.1999.14.7.1175. [DOI] [PubMed] [Google Scholar]
- 122.Laroche M, Moulinier L, Leger P, Lefebvre D, Mazieres B, Boccalon H. Bone mineral decrease in the leg with unilateral chronic occlusive arterial disease. Clin Exp Rheumatol. 2003;21:103–106. [PubMed] [Google Scholar]
- 123.Ramseier E. Arteriosclerotic lesions of the bone arteries. Virchows Arch. 1962;336:77–86. [Google Scholar]
- 124.Sugihara N, Matsuzaki M. The influence of severe bone loss on mitral annular calcification in postmenopausal osteoporosis of elderly Japanese women. Jpn Circ J. 1993;57:14–26. doi: 10.1253/jcj.57.14. [DOI] [PubMed] [Google Scholar]
- 125.Sugihara N, Matsuzaki M, Kato Y. Assessment of the relation between bone mineral metabolism and mitral annular calcification or aortic valve sclerosis--the relation between mitral annular calcification and postmenopausal osteoporosis in elderly patients. Nippon Ronen Igakkai Zasshi- Japanese Journal of Geriatrics. 1990;27:605–615. doi: 10.3143/geriatrics.27.605. [DOI] [PubMed] [Google Scholar]