Abstract
Osteoporosis is a common skeletal disease with a strong genetic component characterized by reduced bone mass and increased risk of fragility fractures. Bone mineral density (BMD) is considered the best established risk factor for osteoporotic fractures.
Over the last years a large number of studies have pointed to the variability in many target genes and their relation with BMD and other determinants of fracture risk such as ultrasound bone properties, skeletal geometry and bone turnover markers. The importance of genetic factors in the bone quality is substantial, but no consensus exists yet on the genes that are involved.
Although osteoporosis is world healthy problem, there are many differences in human ethnics regarding both disease morbidity and drug treatment efficacy. Heterogeneity in drug response may reflect varying responsiveness to osteoporosis treatments due to allele variation in signaling pathway genes such as vitamin D receptor (VDR) or estrogen receptor α (ERα). Polymorphisms of VDR and ERαloci appear genetic determinants of their corresponding hormonal treatment response such as vitamin D and estrogens. Because of their specific ethnic distribution, polymorphisms of VDR and ERαgenes may be involved in reported human differences of osteoporosis treatment responses.
Knowledge of the molecular and functional consequences of the gene polymorphisms is crucial to fully appreciate their significance and understand their potential clinical implications. Future studies and preventive strategies to management osteoporosis need to take in account these genetic factors.
Keywords: genetics, estrogen receptor, osteoporosis, pharmacogenomics, polymorphism, vitamin D receptor
Introduction
Osteoporosis affects an estimated 75 million people among Europe, the United States (US) and Japan (1) and represents a major health problem, especially in countries where life expectancy has dramatically increased during the past decades. Hip and vertebral fractures, which are frequent complications of osteoporosis, represent one of the most important causes of morbidity and mortality among elderly people around the world (2, 3). Bone mineral density (BMD) is the major determinant of fragility fracture (4). Although many environmental factors, such as dietary intakes, physical activities, education, etc., play an important role in BMD, it is strongly inherited. From studies of monozygotic and dizygotic twins, inheritance was estimated to account for 60-80% of BMD in both men (5) and women (6, 7). In this regards, a large number of polymorphisms in multiple candidate genes have been investigated (8). Of them, vitamin D receptor (VDR) and estrogen receptor α (ERα) have been among of the most intensively studied genes in genetic regulation of BMD.
To date, interest of most scientists and clinicians working in genetics, is to arrange genetic markers useful in the patient treatment. In this view, genetics not only offer possibility to anticipate pathological phenotype even before real disease onset but also to foresee the specific patient response to drugs. An early specific and efficacious medicine means greater healthy chances for patient and less hospital economic loss.
The present analysis reviews available molecular data of two major osteoporotic treatments based on vitamin D and estrogens regarding predictor markers for their clinical drug response. Many clinical clues suggest human genetic backgrounds play major role determining treatment effectiveness. Treatment responses to vitamin D or estrogens are affected by specific genotypes of target genes such as VDR and ERα. In our opinion, this review could offer argument of pharmacological data reanalysis and/or new future health strategies.
Vitamin D receptor gene
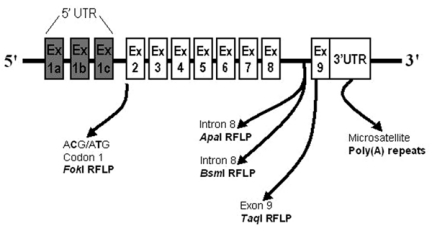
Since 1990s, the gene encoding for VDR was proposed as major genetic locus of bone mass (9). The VDR gene is located on the long arm of chromosome 12 (12q12-14) and is composed by 10 exons, the first of which is not transcribed, and 8 introns (10). The nine coding exons are transcribed into the VDR messenger RNA (mRNA), which in turn is translated into the functional VDR protein. Several restriction fragment length polymorphisms (RFLPs) in the human VDR gene locus have been used in population-based studies (Figure1). The respective restriction endonuclease enzymes have been conventionally indicated with lowercase letter (t, a, borf, respectively for TaqI,ApaI,BsmI andFokI restriction endonucleases), while uppercase letter (T, A, BorF) indicates the absence of the restriction site. TheBsmI andApaI polymorphisms lie in an VDR untranslated region (intron 8) and probably do not confer any functional diversity per se. Similarly, the silent nucleotide substitution in exon 9 that creates theTaqI polymorphism does not affect the amino acid composition of VDR protein (9). Because of their next sites, these VDR 3’end polymorphisms (i.e.BsmI,TaqI andApaI RFLPs) were in linkage disequilibrium such thatAandBalleles were strongly associated withtallele, whileaandballeles with the absence ofTaqI restriction site (Tallele). Morrison et al. (11) used for the first time a candidate gene approach to related commonBsmI allelic variants at VDR 3’end region with bone turnover. Subsequently, the same authors suggested a major contribution by VDR 3’end RFLPs to the BMD genetic determination: VDR gene was originally claimed to contribute to almost 75% of the genetic variation on the BMD (9). In both twin pairs and unrelated post-menopausal women, they showed significantly decreased BMD values at lumbar spine and proximal femur in relation toBsmI Ballele (9). After this original report, conflicting data have been published on the association of the diallelicBsmI RFLP in the VDR locus and BMD both in pre- (12-15) and post-menopausal (16-26) women. Several population studies have essentially confirmed this association, with differences in BMD ranging from 4 to 13% between the oppositeBBandbbgenotypes (14, 16, 17, 22, 23). Other studies found no significant association between VDR alleles and BMD (12, 15, 19, 26), whereas others reported an inverse association to that originally proposed, with subjects with theBBgenotype showing higher and not lower BMD values than thebbgenotype (13, 20, 21). Similarly, studies examining the relationship of this VDR polymorphism with bone turnover markers (12, 19, 24, 27), rates of bone loss (19, 28-30) and osteoporotic fractures (21, 30-35) yielded conflicting results.
Figure 1.
Gene polymorphisms in the human VDR locus potentially involved in vitamin D treatment response.
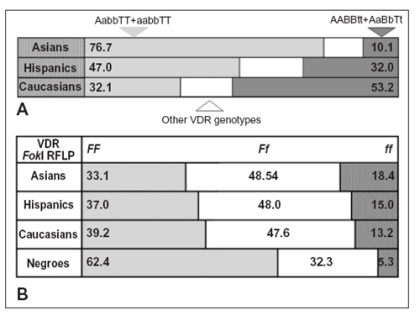
Also using combined VDR RFLP analysis, the found BMD associations were not confirmed in all ethnics around the world. In a large and ethnically homogeneous Caucasian population study (16), a significant segregation of VDR genotypes with lumbar BMD was observed, similar to what previously reported in Australian Caucasians (9, 36). TheAABBttgenotype showed a spinal BMD 13% less thanaabbTTgenotype (16). However, the BMD data were not confirmed by other showing different or no VDR genotype associations with BMD both in Caucasian and Asian populations (19, 20, 37-39). From these population based studies, it is also evident that the VDR polymorphism distribution presents specific ethnic patterns (40). Considering the 4 most frequent genotypes of VDR 3’end, there are 3 specific ethnic patterns (Figure2A). In Asian populations,AabbTTandaabbTTgenotypes (more than 75%) are predominant thanAABBttandAaBbTtgenotypes which are the most frequent in Caucasians (about 53%) (40). Do VDR genotypes influence clinical response to osteoporosis drug treatments? Few studies actually analyzed vitamin D treatment response regarding VDR genotype producing confuse data. At first, Matsuyama et al. (41) assayed VDR genotype response to 1αOHD3treatment (1 mg per day) in an 1-yr retrospective trial based on 120 Japanese osteoporotic women. Although they found only 2BsmIBBgenotypes, the more common genotypes (aabbTTandAabbTT, 75% of subjects) were associated with higher 1αOHD3response thanAABbTtandAaBbTtgenotypes (p<0.001) (41). In a UK twin pairs study, there was a modest trend toward a positive effect of 800 IU D3/day treatment in total hip BMD for the VDRTTgenotype (42). By contrast, the poor response in the genotypeTt, the most common in Caucasians, could account for the generally good responses to vitamin D recorded in Japanese compared to Caucasian subjects (40, 41). Collectively these data could suggest VDRTTgenotype (or linked VDRbbgenotypes) is associated to higher vitamin D response.
Figure 2.
Ethnic frequencies of VDR genotypes detected by ApaI-BsmI-TaqI (A) and FokI (B) endonucleases, respectively. Data modified from ref. 40.
In an Australian women study, Howard et al. (27, 43) reported a greater PTH response in VDRbb genotype vs. BB genotype with short-term calcitriol administration (2 µg of 1,25(OH)2D3/week). However, their original findings of differences in osteocalcin and 1,25(OH)2D3levels between genotypes were not confirmed in the reanalysis (43). Because of VDRB allele is in linkage disequilibrium witht allele in Caucasian populations (38), these VDR data could agree with above TaqI genotype data. By contrast, Graafmans et al. (44) reported that BMD increases in the vitamin D group (400 IU D3/day) relative to the placebo group, was significantly higher in BB (∆BMD 4.4%) and Bb (∆BMD 4.2%) genotype compared with bb genotypes (∆BMD -0.3%). Finally, an probably explanation for the inconsistency regarding which VDR RFLP allele is associated with low BMD response, is that BsmI-TaqI RFLPs do not represent functional loci but are in linkage disequilibrium with a bone-related gene elsewhere.
Because vitamin D and estrogen systems present many crosstalk levels, allelic variants of their signaling pathways could modify mutual hormone response (45-48). In this view, some authors analyzed VDR genotype as response marker to hormone replacement therapy (HRT). A US study based on 108 European Caucasian women, reported BsmIBB genotype was associated with larger spinal BMD increase using low HRT dose whereas VDRbb genotype was associated with larger decrease in the placebo group (49). As previously reported (50) and irrespective for VDRApaI or FokI sites, Japanese women with VDRTT genotype showed significantly higher ∆BMD with HRT than those with Tt genotype (2.6% ± 0.5% vs. –0.8 ± 1.4%; p = 0.016) at 1 year and slightly higher ∆BMD (3.8% ± 0.6% vs. 0.8 ± 1.6%; p = 0.069) at 2 years, but no significant differences between TT and Tt genotypes were seen at 3 years or later (51). Considering that BsmI B allele is TaqIt-allele-linked, these studies produced conflicting data. Giguere et al. (52) found, in a cross-sectional study, that results of quantitative ultrasound examination of the heel in postmenopausal women receiving HRT for more than 5 years were affected by variations in VDR and ERα loci. On the contrary, in a recent Danish study (429 Caucasian women), no VDR genotype effect on changes in bone mass during the subsequent 5 years could be detected, irrespective of HRT (1-2 mg estradiol/day) or not (53). VDRBsmI genotype may be also involved in individual response to cyclic etidronate, raloxifene, alendronate treatments (54, 55). Although the picture is still complicated, there seems to be a trend for the VDRABt haplotype (linked to short poly(A) microsatellite in the 3’UTR, see below) to display somewhoat better responses than the abT haplotype (linked to long poly(A) repeat alleles) (56).
The above data regarding ApaI, BsmI and TaqI RFLPs, suggest that these VDR neutral polymorphisms should therefore be considered as possible markers, in linkage disequilibrium with functional genetic variants affecting structure or expression of VDR gene. 3’untranslated terminal region (3’UTR) of eukaryota genes contain sequence elements regulating mR-NA stability expression (57, 58). The 3’UTRs associated with BsmI-ApaI-TaqI haplotypes, result in substantial differences in VDR gene expression using a reporter gene assay (9); however, the responsible sequence variants have not yet been identified. Therefore, a major confounding factor in VDR studies, could be various linkage patterns of VDR 3’UTR RFLPs present in different human ethnics which confer different associations between BMD and VDR 3’UTR haplotypes (40).
In 1997, Ingles et al. (59) described a polymorphic microsatellite located approximately 1-kb upstream from the VDR 3’UTR. The microsatellite consists of a string of adenosine residues [poly(A) repeats] with polymorphic length varying from 13 to 24 adenosine repeats. Although at least 12 alleles were identified (A13toA24) of VDR poly(A) repeats, allele size follows a bimodal distribution with distinct short (A13-A17) and long (A18A24) allele populations. As shown in figure 3, various distributions of VDR poly(A) repeats have been reported in human ethnic groups (40).
Assuming that VDRBsmIB and b alleles are in disequilibrium with short and long poly(A)alleles respectively, agreement was high in human ethnics though only in Afro-Americans: more than 90% in Asians, 93% in Caucasians and 81% in Hispanics (60). About only 2 VDR 3’UTR haplotypes exist in no-African populations (i.e. Caucasian, Asian and Hispanic subjects): BsmIB allele with short poly(A) repeats (B-short poly(A) haplotype) in contrast to b allele with long poly(A) repeats (b-long poly(A) haplotype) (60). Therefore, BsmI site (and highly linkedTaqI site) is not a good marker of VDR 3’UTR itself, as judged by the poly(A) site (60). Misclassification of 3’UTR poly(A) alleles by BsmI (and also TaqI) site is most severe in African Americans (37%), lesser in Caucasians (7%) and Asians (8,5%) (60).
Figure 3.
Ethnic distribution of VDR poly(A)n repeat polymorphism. Allele cutoffs: short allele (A13-A17 repeats) and long allele (A18-A24 repeats). Data from ref. 60.
Grundberg et al. (61) recently investigated poly(A) microsatellite and linked BsmI site of VDR gene in a population-based cohort of 343 Swedish women aged 20-39. They showed that women with short poly(A) repeats and/or absence of linked BsmI restriction site on both alleles (BBgenotype) have significantly higher BMD (61). Importantly, the regression analysis showed that VDRBsmI genotype was significantly associated with lumbar spine BMD also when taking fat mass, lean mass, height and age into account (p=0.03) (61). The same trend was seen concerning the effect of VDRpoly(A) genotype on lumbar spine BMD after adjustments (p=0.06) (61).
If ApaI-BsmI-TaqI polymorphisms are not probably functional but disequilibrium marker liked to other VDR polymorphisms, which is the molecular effect of poly(A) repeats on the VDR function? Long poly(A) allele (A18-A24 repeats), displays higher vitamin D-induced transcriptional activity than short poly(A) allele (A13-A17 repeats), although it does not achieve statistical significance (62). Since poly(A) polymorphism occurs in exon 9, but is expressed only in the 3’UTR of VDR mRNA, long poly(A) allele may produce VDR mRNA that is more stable and/or is translated more efficiently into protein than short allele. Other data using RFLP approach, agree with this hypothesis (9, 62-66). Interestingly, high presence of long poly(A) allele in Asians than others (60), may be involved in the suggested higher “vitamin D-sensibility” of Asians than VDR RFLPs (i.e.ApaI-BsmI-TaqI or FokI RFLPs) (40-41). However, as clearly shown by Haussler’s group, simultaneous analysis of poly(A) repeats and FokI RFLPs is needed to determine the specific effects of VDR variants on the overall VDR function (62).
Regarding VDRFokI site, F allele produces a more active VDR protein than f allele (62, 67-69) and F and long poly(A) alleles have synergic effect increasing VDR protein activity (62). Data indicating a more active VDRF allele is consistent with many clinical studies which suggest F allele (vs. opposite f allele) is associated with increased BMD (67, 70-74), higher rates of bone turnover (50), lower risk for primary hyperparathyroidism (75, 76), lower risk for intervertebral disc degeneration (77) and lower incidence of vertebral fracture (78). Figure 2B showed VDRFokI genotype distribution regarding ethnic groups around the world. Because F allele is more represented in Caucasians than Asians, its genetic effects may contrast those of poly(A) polymorphism (40).
The above data suggest that the assaying of genotypic effects on the VDR function, needs a simultaneous analysis of all functional variants (i.e. FokI RFLP, poly(A) repeats and Cdx-2 polymorphisms (79)) and not only of the disequilibrium markers (i.e. ApaI-BsmI-TaqI RFLPs) which are frequently ethnic-dependent.
Estrogen receptor alpha gene
The genes encoding estrogen receptors, ERα and ERβ, have been considered as important candidate markers of osteoporotic risk. The importance of ERα gene in the bone tissue has been indicated by the osteoporotic phenotype in a man with a nonsense mutation in the ERα gene (80) as well as the reduced BMD values in mice lacking a functional ERα gene (81, 82), but not in those lacking ERβ (82, 83), strongly proposing ERα gene as major mediator of estrogen response at least in bone system.
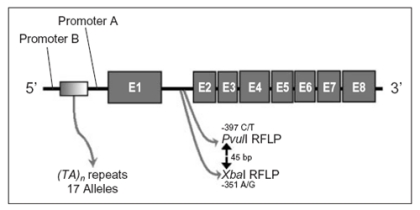
The human ERα gene is located on the chromosome 6p25.1, comprises eight exons, and spans more than 140 kb (84). Actually, some ERα polymorphisms are proposed as involved in the bone system (Figure4). Two single nucleotide polymorphisms have been identified in intron 1 of ERα gene: a T-397C polymorphism that is recognized by the restriction endonuclease PvuII [T and C alleles correspond to the presence (p allele) and absence (P allele) of the PvuII restriction site, respectively] and an A-351G polymorphism that is recognized by XbaI [A and G alleles correspond to the presence (x allele) and absence (Xallele) of the XbaI restriction site, respectively] (40). These ERα intron 1 RFLPs, alone or in combination, have been associated with bone mass in post-menopausal women (85-89) or pre-menopausal women (90, 91). However, other studies have not confirmed these observations (16, 92-96). In addition, a microsatellite (TA)n repeats polymorphism, located in ERα promoter area and strongly linked to PvuII-XbaI sites (93), was associated with BMD and with the prevalence of fractures (86, 93-94).
Figure 4.
Gene polymorphisms in the human ERα locus potentially involved in estrogen treatment response.
Actually, few studies analyzed ERα polymorphisms related to HRT responsiveness. In Korean post-menopausal women, Han et al. (96) found no significant effects of ERα intron 1 genotypes on the BMD and on the HRT responsiveness. Similarly, in a Japanese study, although ERαPP genotypes had significantly higher spine BMD than Pp and pp genotypes, no significant BMD change between ERα genotypes were present after 1-yr-HRT treatment [0.625 mg conjugated equine estrogens (CEE) plus 2.5-5 mg medroxyprogesterone acetate [MPA]/daily) (49). Also, XbaI variants were not associated spinal BMD with and without HRT treatment (49). Another Japanese study recently reported that response to HRT (0.625 mg CEE or 2 mg transdermal estradiol) in postmenopausal period were greater in pp genotype than in women with other PvuII genotypes only within first 6 HRT months (97). The significant difference in the BMD gain observed at 6 months, was not confirmed after 12 months of HRT. In addition, there were no significant difference related to XbaI genotypes (97).
In a study based on different Asian genetic background (Thai women), Ongphiphadhanakul et al. (98) reported that women on 0.3 mg CEE with P allele (PP and Pp genotypes) had significantly higher increase in lumbar spinal BMD compared to those without P allele (pp genotype) after 1-year treatment (p<0.05). No difference in the BMD change at femoral neck was found on 0.3 mg CEE treatment. Neither the changes in vertebral nor femoral BMD were different among subjects on 0.625 mg CEE with different PvuII genotypes (98). These Thai data were consistent with a Caucasian elderly women study, which suggested a relationship between BMD changes after low estrogen dose replacement (0.3 mg CEE/day) and ERα genotypes (48). For 3.5 years period of HRT treatment, BMD changes were analyzed at the spine, femoral neck, distal radius and total body BMC. Where ERα genotypic effects were significant, PP (or xx) genotype was generally associated with larger decreases (or smaller increase) of bone mass, whereas pp (or XX) genotype was associated with smaller decreases (or larger increase) of bone mass on HRT treatment (48). Recently, Rapuri et al. (99) evaluated the influence of ERα intron 1 RFLPs in 79 postmenopausal women receiving HRT (0.625 mg CEE plus 2.5 mg MPA) for 3 years. The percent change in BMD was higher in women with ERα genotype, XX or PP compared to women with ERα genotype xx or pp but was significant only for total body of PvuII genotype PP. Collectively the above data suggest that ERαpp genotype is a relatively estrogen-insensible genotype, and that women with P allele (Pp and PP genotypes) benefit more from the protective effect of HRT on fracture risk than women with pp genotype, though long-term HRT seemed to eliminated the ERα genotype related differences in the BMD (100, 101).
Regarding other estrogen-sensible tissues, Herrington and colleagues (102, 103) studied ERα RFLP effects in women affected by coronary artery disease. As major results, they reported statistical associations between clinical parameters (i.e. response of E-selectin and of HDL-cholesterol to HRT) and women with PP genotype than the opposite pp genotype (102, 103). PP genotype had greater increase in the HDL-cholesterol (and also decrease in the E-selectin) level by HRT (0.625 mg CEE/day) than level changes observed in other PvuII genotypes. Similar patterns of response were observed for XbaI polymorphism (102, 103).
The functional significance of PvuII-XbaI polymorphisms, however, is not clear: PvuIIP allele disrupts a potential recognition site for the transcriptional factor AP4 which recognizes CAGCTG sequence. Three studies recently detected enhancer activities in the ERα intron 1. These enhancer activities differed among PvuII-XbaI RFLPs: P allele confers higher transcriptional activity than p allele in gene reporter constructs (102, 104, 105).
Finally, all the above studies based on different ethnic populations (Asians and Caucasians) suggested that ERαP allele confers relatively estrogen-resistant than the opposite p allele using low estrogen dose (equivalent to 0.3 mg CEE/day). Using higher estrogen dose, these PvuII genotype effect seems to disappear at least in skeleton system while in cardiovascular systems, a different genetic response regarding ERα intron 1 polymorphisms could persist even using higher estrogen doses than 0.3 mg CEE.
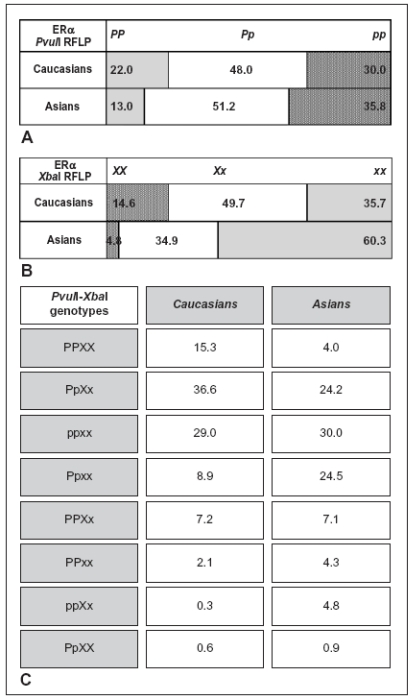
As shown in figure5A, because estrogen-sensible PvuIIP allele is more represented in Caucasians than Asians, it supports the clinical feeling of an higher Caucasian responsiveness to estrogen treatments than Asians (40). On the other hand, the relatively estrogen-resistance of Asian background may be a selection force consequence: because Asians meanly present higher exposure to environmental estrogens, such as soy dietary phytoestrogens, the selection of more estrogen-resistant genotype (i.e. ERαpp genotype) could represent a defensive mechanism against environment (106-108). ERαXbaI polymorphism also presents different genotype distribution in human populations with x allele more represented in Asians than Caucasians (Figure5B). This does not surprise because the strong and highly significant linkage disequilibrium between PvuII and XbaI sites as expected for two sites separated by 45 bp (40, 93). Generally, P and X alleles, as well as p and x alleles were strongly associated with each other although combined PvuII-XbaI genotype frequencies vary regard to different ethnics (Figure5C). The haplotype pX was not observed in the majority of studies, were haplotype Px was detected even though at low frequency suggesting the disequilibrium is not complete and either recombination or multiple mutations have occurred between or at these two polymorphic restriction sites (40).
Figure 5.
Ethnic frequencies of ERα genotypes detected by PvuII (A) and XbaI (B) endonucleases, and of the 8 most frequent PvuII-XbaI genotypes (C), respectively. Data modified from ref. 40.
ERα microsatellite (TA)n repeats proposed as strong risk marker of postmenopausal osteoporosis, may be also involved in the estrogen-sensibility. Indeed, for clinical data and for their position in promoter area, (TA)n repeat polymorphisms could be functional modifying ERα expression and/or regulation (93). In addiction, Yim et al. (109) recently reported the percent change of lumbar spine BMD after 1-yr HRT (0.625 mg CEE with/without 2.5 mg MPA) significantly decreased (r = –0.131; p = 0.035) with an increase in the mean number of (TA)n repeats.
As represented in figure 6, similar (TA)n repeat frequencies were reported in Asian and Caucasian studies with comparative bimodal distributions peaked around 12-16 and 22-24 (TA)n repeats (93, 94, 98, 110-114). In contrast to ERα intron 1 RFLPs, (TA)n repeat frequencies do not apparently differ in Asians vs. Caucasians suggesting a lesser involvement in the specific ethnic pharmaco-sensitivity. However, more studies are needed to confirm or refuse this issue.
Figure 6.
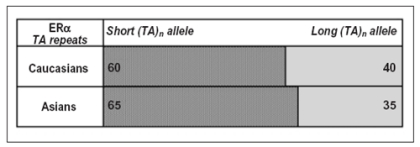
Ethnic distribution of ERα (TA)n repeat polymorphism located in the human ERα promoter area. Allele cutoffs: short (TA)n repeat allele (<TA18 repeats) and long (TA)n repeat allele (≥ TA18 repeats). Data modified from ref. 40.
Clinically detected effects of ERα polymorphisms (i.e. PvuII-XbaI RFLP and (TA)n repeats) are likely dependent, at least in part, to linkage disequilibrium each other and with other functional nucleotide changes in the nearby exons of ERα gene or its 5’ regulatory sequence. This could be major confounding factor in the ERα polymorphism studies. In this view, a synonymous nucleotide substitution from T to C at codon 10 of ERα exon 1, was in linkage disequilibrium with intron 1PvuII RFLPs (115). The C262 allele appeared in linkage with P allele of the intronic polymorphism. After treating 96 post-menopausal women with 0.3-0.625 mg CEE for 2 years, vertebral BMD increased regardless of the T262C genotype. However, with regard to femoral neck BMD, only those subjects that were homozygous for T262C polymorphism had an increase in femoral BMD (115).
T262C polymorphism at the ERα locus, may represent another level of genetic modulation of estrogen responsiveness. However, it is unclear how the synonymous nucleotide change could influence the ERα function. One of the possibilities is that T262C polymorphism may affect an alternative translation initiation site. Generally the ATG codon with appropriate context nearest the 5’end of the mRNA serves as the initiation codon (116) and a polymorphism of nucleotide sequence around the initiation codon influences the surface levels of cell adhesion receptors (117). Occasional escape from this first-ATG rule occurs. The ERα T262C polymorphism is located 29 nucleotides downstream from the putative translation site in the vicinity of another ATG codon around which the context GCATC[T/C]GGGATGG may be appropriate for it to serve as another translation initiation site. So, T262C variants may influence the favorableness of its being an alternative start codon (115). More studies regarding this issue are needed.
In conclusion, none of ERα allelic variations could completely value the estrogen pharmacogenetics or heredity of complex trait estrogen dependent such as BMD. As suggest Haussler’s group data on VDR variants (62), only simultaneous analysis of all alleles (i.e. PvuII-XbaI RFLPs, T262C, (TA)n repeats and others) present in ERα locus may offer an understanding of phenotypic heredity regarding the ERα function.
Conclusion
It is well recognized that different patient respond in different ways to the same medication. These differences are often greater among members of a population than they are within the same person at different times (or between monozygotic twins) (118). The existence of large population differences with small intrapatient variability is consistent with inheritance as determinant of drug response; it is estimated that genetics can account for 20 to 95% of variability in drug disposition and effects (119). Although many nongenetic factors influence the effects of medications, including age, organ function, concomitant therapy, drug interactions, and the nature of the disease, there are now numerous examples of cases in which interindividual differences in drug response are due to sequence variants in genes encoding drug-metabolizing enzymes, drug transporters or drug targets (120). Unlike other factors influencing drug response, inherited determinants generally remain stable throughout a person’s lifetime.
Clinical observations of inherited differences in drug effects were first documented in the 1950s (121, 122), giving rise to the field of pharmacogenetics, and later pharmacogenomics. Although the two terms are synonymous for all practical purposes, pharmacogenomics uses genome-wide approaches to elucidate the inherited basis of differences between persons in the response to drugs.
The potential implication of pharmacogenomics in clinical research and clinical medicine is that disease could be treated according to genetic and specific individual markers, selecting medications and dosages that are optimized for individual patients (“the right drug into the right patient”). The possibility of defining patient populations genetically may improve outcomes by predicting individual responses to drugs, and could improve therapy safety and efficacy. This personalizing of medicines has been the holy grail of pharmacogenomics since sequencing the human genome was conceptualized. The application of genomic technologies, such as gene sequencing, statistical genetics and gene expression analysis to drug development, holds great promise for the future of medicine. Unfortunately, our ability to identify patients at risk for disease, stratify patients by clinical outcome and treatment response or predict adverse event occurrences is, in reality, several years away.
References
- 1.Nevitt MC. Epidemiology of osteoporosis. Rheum Dis Clin North Am. 1994;20:535–59. [PubMed] [Google Scholar]
- 2.Keene GS, Parker MJ, Proyor GA. Morbidity and mortality after hip fractures. BMJ. 1993;307:1248–50. doi: 10.1136/bmj.307.6914.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Center JR, Nguyen TV, Schneider D, et al. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353:878–82. doi: 10.1016/S0140-6736(98)09075-8. [DOI] [PubMed] [Google Scholar]
- 4.Dalen N, Hellstrom LG, Jacobson B. Bone mineral content and mechanical strength of the femoral neck. Acta Orthop Scand. 1976;47:503–8. doi: 10.3109/17453677608988728. [DOI] [PubMed] [Google Scholar]
- 5.Christian JC, Yu PL, Slemenda CW, et al. Heritability of bone mass: a longitudinal study in aging male twins. Am J Hum Genet. 1989;44:429–33. [PMC free article] [PubMed] [Google Scholar]
- 6.Pocock NA, Eisman JA, Hopper JL, et al. Genetic determinants of bone mass in adults. A twin study. J Clin Invest. 1987;80:706–10. doi: 10.1172/JCI113125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slemenda CW, Christian JC, Williams CJ, et al. Genetic determinants of bone mass in adult women: a reevaluation of the twin model and the potential importance of gene interaction on heritability estimates. J Bone Miner Res. 1991;6:561–7. doi: 10.1002/jbmr.5650060606. [DOI] [PubMed] [Google Scholar]
- 8.Massart F, Reginster JY, Brandi ML. Genetics of menopause-associated diseases. Maturitas. 2001;40:103–16. doi: 10.1016/s0378-5122(01)00283-3. [DOI] [PubMed] [Google Scholar]
- 9.Morrison NA, Qi JC, Tokita A, et al. Prediction of bone density from vitamin D receptor alleles. Nature. 1994;367:284–7. doi: 10.1038/367284a0. [DOI] [PubMed] [Google Scholar]
- 10.Haussler MR, Whitfield GK, Haussler CA, et al. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13:325–49. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- 11.Morrison NA, Yeoman R, Kelly PJ, et al. Contribution of trans-acting factor alleles to normal physiological variability: vitamin D receptor gene polymorphism and circulating osteocalcin. Proc Natl Acad Sci USA. 1992;89:6665–9. doi: 10.1073/pnas.89.15.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garnero P, Borel O, Sornay-Rendu E, et al. Vitamin D receptor gene polymorphisms do not predict bone turnover and bone mass in healthy premenopausal women. J Bone Miner Res. 1995;10:1283–8. doi: 10.1002/jbmr.5650100902. [DOI] [PubMed] [Google Scholar]
- 13.Salamone LM, Ferrell R, Black DM, et al. The association between vitamin D receptor gene polymorphisms and bone mineral density at the spine, hip and whole-body in premenopausal women. Osteoporos Int. 1996;6:63–8. doi: 10.1007/BF01626540. [DOI] [PubMed] [Google Scholar]
- 14.Tokita A, Matsumoto H, Morrison NA, et al. Vitamin D receptor alleles, bone mineral density and turnover in premenopausal Japanese women. J Bone Miner Res. 1996;11:1003–9. doi: 10.1002/jbmr.5650110718. [DOI] [PubMed] [Google Scholar]
- 15.Hansen TS, Abrahamsen B, Henriksen FL, et al. Vitamin D receptor alleles do not predict bone mineral density or bone loss in Danish perimenopausal women. Bone. 1998;22:571–5. doi: 10.1016/s8756-3282(98)00028-3. [DOI] [PubMed] [Google Scholar]
- 16.Gennari L, Becherini L, Masi L, et al. Vitamin D and estrogen receptor allelic variants in Italian postmenopausal women: evidence of multiple gene contribution to bone mineral density. J Clin Endocrinol Metab. 1998;83:939–44. doi: 10.1210/jcem.83.3.4649. [DOI] [PubMed] [Google Scholar]
- 17.Spector TD, Keen RW, Arden NK, et al. Influence of vitamin D receptor genotype on bone mineral density in postmenopausal women: a twin study in Britain. BMJ. 1995;310:1357–60. doi: 10.1136/bmj.310.6991.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riggs BL, Nguyen TV, Melton LJ 3rd, et al. The contribution of vitamin D receptor gene alleles to the determination of bone mineral density in normal and osteoporotic women. J Bone Miner Res. 1995;10:991–6. doi: 10.1002/jbmr.5650100622. [DOI] [PubMed] [Google Scholar]
- 19.Garnero P, Borel O, Sornay-Rendu E, et al. Vitamin D receptor gene polymorphisms are not related to bone turnover, rate of bone loss, and bone mass in postmenopausal women: the OFELY Study. J Bone Miner Res. 1996;11:827–34. doi: 10.1002/jbmr.5650110614. [DOI] [PubMed] [Google Scholar]
- 20.Uitterlinden AG, Pols HA, Burger H, et al. A large-scale population-based study of the association of vitamin D receptor gene polymorphisms with bone mineral density. J Bone Miner Res. 1996;11:1241–8. doi: 10.1002/jbmr.5650110908. [DOI] [PubMed] [Google Scholar]
- 21.Houston LA, Grant SF, Reid DM, et al. Vitamin D receptor polymorphism, bone mineral density, and osteoporotic vertebral fracture: studies in a UK population. Bone. 1996;18:249–52. doi: 10.1016/8756-3282(95)00483-1. [DOI] [PubMed] [Google Scholar]
- 22.Vandevyver C, Wylin T, Cassiman JJ, et al. Influence of the vitamin D receptor gene alleles on bone mineral density in postmenopausal and osteoporotic women. J Bone Miner Res. 1997;12:241–7. doi: 10.1359/jbmr.1997.12.2.241. [DOI] [PubMed] [Google Scholar]
- 23.Tamai M, Yokouchi M, Komiya S, et al. Correlation between vitamin D receptor genotypes and bone mineral density in Japanese patients with osteoporosis. Calcif Tissue Int. 1997;60:229–32. doi: 10.1007/s002239900219. [DOI] [PubMed] [Google Scholar]
- 24.Zmuda JM, Cauley JA, Danielson ME, et al. Vitamin D receptor gene polymorphisms, bone turnover, and rates of bone loss in older African-American women. J Bone Miner Res. 1997;12:1446–52. doi: 10.1359/jbmr.1997.12.9.1446. [DOI] [PubMed] [Google Scholar]
- 25.Sigurdsson G, Magnusdottir DN, Kristinsson JO, et al. Association of BsmI vitamin-D receptor gene polymorphism with combined bone mass in spine and proximal femur in Icelandic women. J Intern Med. 1997;241:501–5. doi: 10.1111/j.1365-2796.1997.tb00008.x. [DOI] [PubMed] [Google Scholar]
- 26.Jorgensen HL, Scholler J, Sand JC, et al. Relation of common allelic variation at vitamin D receptor locus to bone mineral density and postmenopausal bone loss: cross sectional and longitudinal population study. BMJ. 1996;313:586–90. doi: 10.1136/bmj.313.7057.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howard G, Nguyen T, Morrison N, et al. Genetic influences on bone density: physiological correlates of vitamin D receptor gene alleles in premenopausal women. J Clin Endocrinol Metab. 1995;80:2800–5. doi: 10.1210/jcem.80.9.7673427. [DOI] [PubMed] [Google Scholar]
- 28.Keen RW, Major PJ, Lanchbury JS, et al. Vitamin-D-receptor-gene polymorphism and bone loss. Lancet. 1995;345:990. [PubMed] [Google Scholar]
- 29.Kikuchi R, Uemura T, Gorai I, et al. Early and late postmenopausal bone loss is associated with BsmI vitamin D receptor gene polymorphism in Japanese women. Calcif Tissue Int. 1999;64:102–6. doi: 10.1007/s002239900586. [DOI] [PubMed] [Google Scholar]
- 30.Gomez C, Naves ML, Barrios Y, et al. Vitamin D receptor gene polymorphisms, bone mass, bone loss and prevalence of vertebral fracture: differences in postmenopausal women and men. Osteoporos Int. 1999;10:175–82. doi: 10.1007/s001980050213. [DOI] [PubMed] [Google Scholar]
- 31.Langdahl BL, Gravholt CH, Brixen K, et al. Polymorphisms in the vitamin D receptor gene and bone mass, bone turnover and osteoporotic fractures. Eur J Clin Invest. 2000;30:608–17. doi: 10.1046/j.1365-2362.2000.00686.x. [DOI] [PubMed] [Google Scholar]
- 32.Berg JP, Falch JA, Haug E. Fracture rate, pre- and postmenopausal bone mass and early and late postmenopausal bone loss are not associated with vitamin D receptor genotype in a high-endemic area of osteoporosis. Eur J Endocrinol. 1996;135:96–100. doi: 10.1530/eje.0.1350096. [DOI] [PubMed] [Google Scholar]
- 33.Yanagi H, Tomura S, Kawanami K, et al. Vitamin D receptor gene polymorphisms are associated with osteoporosis in Japanese women. J Clin Endocrinol Metab. 1996;81:4179–81. doi: 10.1210/jcem.81.11.8923886. [DOI] [PubMed] [Google Scholar]
- 34.Aerssens J, Dequeker J, Peeters J, et al. Polymorphisms of the VDR, ER and COLIA1 genes and osteoporotic hip fracture in elderly postmenopausal women. Osteoporos Int. 2000;11:583–91. doi: 10.1007/s001980070079. [DOI] [PubMed] [Google Scholar]
- 35.Uitterlinden AG, Weel AE, Burger H, et al. Interaction between the vitamin D receptor gene and collagen type Ialpha1 gene in susceptibility for fracture. J Bone Miner Res. 2001;16:379–85. doi: 10.1359/jbmr.2001.16.2.379. [DOI] [PubMed] [Google Scholar]
- 36.Morrison NA, Tokita A, Cheng QJ, et al. Allelic variation in the vitamin D receptor gene: major haplotypes in Caucasians. Bone. 1995;16:105S. [Google Scholar]
- 37.Fountas L, Moutsatsou P, Kastanias I, et al. The contribution of vitamin D receptor gene polymorphisms in osteoporosis and familial osteoporosis. Osteoporos Int. 1999;10:392–8. doi: 10.1007/s001980050245. [DOI] [PubMed] [Google Scholar]
- 38.Gennari L, Becherini L, Falchetti A, et al. Genetics of osteoporosis: role of steroid hormone receptor gene polymorphisms. J Steroid Biochem Mol Biol. 2002;81:1–24. doi: 10.1016/s0960-0760(02)00043-2. [DOI] [PubMed] [Google Scholar]
- 39.Kim JG, Kwon JH, Kim SH, et al. Association between vitamin D receptor haplotypes and bone mass in postmenopausal Korean women. Am J Obstet Gynecol. 2003;189:1234–1240. doi: 10.1067/s0002-9378(03)00650-1. [DOI] [PubMed] [Google Scholar]
- 40.Massart F. Human races and pharmacogenomics of effective bone treatments. Gynecol Endocr. 2005;20:36–44. doi: 10.1080/09513590400019437. [DOI] [PubMed] [Google Scholar]
- 41.Matsuyama T, Ishii S, Tokita A, et al. Vitamin D receptor genotypes and bone mineral density. Lancet. 1995;345:1238–9. [PubMed] [Google Scholar]
- 42.Hunter D, Major P, Arden N, et al. A randomized controlled trial of vitamin D supplementation on preventing postmenopausal bone loss and modifying bone metabolism using identical twin pairs. J Bone Miner Res. 2000;15:2276–83. doi: 10.1359/jbmr.2000.15.11.2276. [DOI] [PubMed] [Google Scholar]
- 43.Howard G, Nguyen T, Morrison N, et al. Genetic influences on bone density: physiological correlates of vitamin D receptor gene alleles in premenopausal women. Notification of genotype corrections. J Clin Endocrinol Metab. 1998;83:1043. doi: 10.1210/jcem.83.3.4668-5. [DOI] [PubMed] [Google Scholar]
- 44.Graafmans WC, Lips P, Ooms ME, et al. The effect of vitamin D supplementation on the bone mineral density of the femoral neck is associated with vitamin D receptor genotype. J Bone Miner Res. 1997;12:1241–5. doi: 10.1359/jbmr.1997.12.8.1241. [DOI] [PubMed] [Google Scholar]
- 45.Byrne IM, Flanagan L, Tenniswood MP, et al. Identification of a hormone-responsive promoter immediately upstream of exon 1c in the human vitamin D receptor gene. Endocrinology. 2000;141:2829–36. doi: 10.1210/endo.141.8.7618. [DOI] [PubMed] [Google Scholar]
- 46.van Hoof HJ, van der Mooren MJ, Swinkels LM, et al. Female sex hormone replacement therapy increases serum free 1,25-dihydroxyvitamin D3: a 1-year prospective study. Clin Endocrinol. 1999;50:511–6. doi: 10.1046/j.1365-2265.1999.00693.x. [DOI] [PubMed] [Google Scholar]
- 47.Swami S, Krishnan AV, Feldman D. 1alpha,25-Dihydroxyvitamin D3 down-regulates estrogen receptor abundance and suppresses estrogen actions in MCF-7 human breast cancer cells. Clin Cancer Res. 2000;6:3371–9. [PubMed] [Google Scholar]
- 48.Kinuta K, Tanaka H, Moriwake T, et al. Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology. 2000;141:1317–24. doi: 10.1210/endo.141.4.7403. [DOI] [PubMed] [Google Scholar]
- 49.Deng HW, Li J, Li JL, et al. Change of bone mass in postmenopausal Caucasian women with and without hormone replacement therapy is associated with vitamin D receptor and estrogen receptor genotypes. Hum Genet. 1998;103:576–85. doi: 10.1007/s004390050872. [DOI] [PubMed] [Google Scholar]
- 50.Kurabayashi T, Tomita M, Matsushita H, et al. Association of vitamin D and estrogen receptor gene polymorphism with the effect of hormone replacement therapy on bone mineral density in Japanese women. Am J Obstet Gynecol. 1999;180:1115–20. doi: 10.1016/s0002-9378(99)70603-4. [DOI] [PubMed] [Google Scholar]
- 51.Kurabayashi T, Matsushita H, Tomita M, et al. Association of vitamin D and estrogen receptor gene polymorphism with the effects of longterm hormone replacement therapy on bone mineral density. J Bone Miner Metab. 2004;22:241–247. doi: 10.1007/s00774-003-0474-y. [DOI] [PubMed] [Google Scholar]
- 52.Giguere Y, Dodin S, Blanchet C, et al. The association between heel ultrasound and hormone replacement therapy is modulated by a two-locus vitamin D and estrogen receptor genotype. J Bone Miner Res. 2000;15:1076–1084. doi: 10.1359/jbmr.2000.15.6.1076. [DOI] [PubMed] [Google Scholar]
- 53.Tofteng CL, Jensen JE, Abrahamsen B, et al. Two polymorphisms in the vitamin D receptor gene--association with bone mass and 5year change in bone mass with or without hormone-replacement therapy in postmenopausal women: the Danish Osteoporosis Prevention Study. J Bone Miner Res. 2002;17:1535–44. doi: 10.1359/jbmr.2002.17.8.1535. [DOI] [PubMed] [Google Scholar]
- 54.Palomba S, Orio F Jr, Russo T, et al. BsmI vitamin D receptor genotypes influence the efficacy of antiresorptive treatments in postmenopausal osteoporotic women. A 1-year multicenter, randomized and controlled trial. Osteoporos Int. 2005;16.943-952 doi: 10.1007/s00198-004-1800-5. [DOI] [PubMed] [Google Scholar]
- 55.Marc J, Prezelj J, Komel R, et al. VDR genotype and response to etidronate therapy in late postmenopausal women. Osteoporos Int. 1999;10:303–6. doi: 10.1007/s001980050231. [DOI] [PubMed] [Google Scholar]
- 56.Uitterlinden AG, Fang Y, van Meurs JB. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338:143–146. doi: 10.1016/j.gene.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 57.Sachs AB. Messenger RNA degradation in eukaryotes. Cell. 1993;74:413–21. doi: 10.1016/0092-8674(93)80043-e. [DOI] [PubMed] [Google Scholar]
- 58.Beelman CA, Parker R. Degradation of mRNA in eukaryotes. Cell. 1995;81:179–83. doi: 10.1016/0092-8674(95)90326-7. [DOI] [PubMed] [Google Scholar]
- 59.Ingles SA, Ross RK, Yu MC, et al. Association of prostate cancer risk with genetic polymorphisms in vitamin D receptor and androgen receptor. J Natl Cancer Inst. 1997;89:166–70. doi: 10.1093/jnci/89.2.166. [DOI] [PubMed] [Google Scholar]
- 60.Ingles SA, Haile RW, Henderson BE, et al. Strength of linkage disequilibrium between two vitamin D receptor markers in five ethnic groups: implications for association studies. Cancer Epidemiol Biomarkers Prev. 1997;6:93–8. [PubMed] [Google Scholar]
- 61.Grundberg E, Brändström H, Ribom EL, et al. A poly adenosine repeat in the human vitamin D receptor gene is associated with bone mineral density in young Swedish women. Calcif Tissue Int. 2003;73:455–462. doi: 10.1007/s00223-002-0032-y. [DOI] [PubMed] [Google Scholar]
- 62.Whitfield GK, Remus LS, Jurutka PW, et al. Functionally relevant polymorphisms in the human nuclear vitamin D receptor gene. Mol Cell Endocrinol. 2001;177:145–59. doi: 10.1016/s0303-7207(01)00406-3. [DOI] [PubMed] [Google Scholar]
- 63.Mocharla H, Butch AW, Pappas AA, et al. Quantification of vitamin D receptor mRNA by competitive polymerase chain reaction in PBMC: lack of correspondence with common allelic variants. J Bone Miner Res. 1997;12:726–33. doi: 10.1359/jbmr.1997.12.5.726. [DOI] [PubMed] [Google Scholar]
- 64.Verbeek W, Gombart AF, Shiohara M, et al. Vitamin D receptor: no evidence for allele-specific mRNA stability in cells which are heterozygous for the Taq I restriction enzyme polymorphism. Biochem Biophys Res Commun. 1997;238:77–80. doi: 10.1006/bbrc.1997.7239. [DOI] [PubMed] [Google Scholar]
- 65.Carling T, Rastad J, Akerstrom G, et al. Vitamin D receptor (VDR) and parathyroid hormone messenger ribonucleic acid levels correspond to polymorphic VDR alleles in human parathyroid tumors. J Clin Endocrinol Metab. 1998;83:2255–9. doi: 10.1210/jcem.83.7.4862. [DOI] [PubMed] [Google Scholar]
- 66.Gross C, Krishnan AV, Malloy PJ, et al. The vitamin D receptor gene start codon polymorphism: a functional analysis of FokI variants. J Bone Miner Res. 1998;13:1691–9. doi: 10.1359/jbmr.1998.13.11.1691. [DOI] [PubMed] [Google Scholar]
- 67.Arai H, Miyamoto K, Taketani Y, et al. A vitamin D receptor gene polymorphism in the translation initiation codon: effect on protein activity and relation to bone mineral density in Japanese women. J Bone Miner Res. 1997;12:915–21. doi: 10.1359/jbmr.1997.12.6.915. [DOI] [PubMed] [Google Scholar]
- 68.Jurutka PW, Remus LS, Whitfield GK, et al. The polymorphic N terminus in human vitamin D receptor isoforms influences transcriptional activity by modulating interaction with transcription factor IIB. Mol Endocrinol. 2000;14:401–20. doi: 10.1210/mend.14.3.0435. [DOI] [PubMed] [Google Scholar]
- 69.Colin EM, Weel AE, Uitterlinden AG, et al. Consequences of vitamin D receptor gene polymorphisms for growth inhibition of cultured human peripheral blood mononuclear cells by 1, 25-dihydroxyvitamin D3. Clin Endocrinol. 2000;52:211–6. doi: 10.1046/j.1365-2265.2000.00909.x. [DOI] [PubMed] [Google Scholar]
- 70.Gross C, Eccleshall TR, Malloy PJ, et al. The presence of a polymorphism at the translation initiation site of the vitamin D receptor gene is associated with low bone mineral density in postmenopausal Mexican-American women. J Bone Miner Res. 1996;11:1850–5. doi: 10.1002/jbmr.5650111204. [DOI] [PubMed] [Google Scholar]
- 71.Harris SS, Eccleshall TR, Gross C, et al. The vitamin D receptor start codon polymorphism (FokI) and bone mineral density in premenopausal American black and white women. J Bone Miner Res. 1997;12:1043–8. doi: 10.1359/jbmr.1997.12.7.1043. [DOI] [PubMed] [Google Scholar]
- 72.Tao C, Yu T, Garnett S, et al. Vitamin D receptor alleles predict growth and bone density in girls. Arch Dis Child. 1998;79:488–494. doi: 10.1136/adc.79.6.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ferrari S, Manen D, Bonjour JP, et al. Bone mineral mass and calcium and phosphate metabolism in young men: relationships with vitamin D receptor allelic polymorphisms. J Clin Endocrinol Metab. 1999;84:2043–8. doi: 10.1210/jcem.84.6.5790. [DOI] [PubMed] [Google Scholar]
- 74.Lucotte G, Mercier G, Burckel A. The vitamin D receptor FokI start codon polymorphism and bone mineral density in osteoporotic postmenopausal French women. Clin Genet. 1999;56:221–4. doi: 10.1034/j.1399-0004.1999.560307.x. [DOI] [PubMed] [Google Scholar]
- 75.Correa P, Rastad J, Schwarz P, et al. The vitamin D receptor (VDR) start codon polymorphism in primary hyperparathyroidism and parathyroid VDR messenger ribonucleic acid levels. J Clin Endocrinol Metab. 1999;84:1690–4. doi: 10.1210/jcem.84.5.5707. [DOI] [PubMed] [Google Scholar]
- 76.Sosa M, Torres A, Martin N, et al. The distribution of two different vitamin D receptor polymorphisms (BsmI and start codon) in primary hyperparathyroidism. J Intern Med. 2000;247:124–30. doi: 10.1046/j.1365-2796.2000.00593.x. [DOI] [PubMed] [Google Scholar]
- 77.Videman T, Leppavuori J, Kaprio J, et al. Intragenic polymorphisms of the vitamin D receptor gene associated with intervertebral disc degeneration. Spine. 1998;23:2477–85. doi: 10.1097/00007632-199812010-00002. [DOI] [PubMed] [Google Scholar]
- 78.Gennari L, Becherini L, Mansani R, et al. FokI polymorphism at translation initiation site of the vitamin D receptor gene predicts bone mineral density and vertebral fractures in postmenopausal Italian women. J Bone Miner Res. 1999;14:1379–86. doi: 10.1359/jbmr.1999.14.8.1379. [DOI] [PubMed] [Google Scholar]
- 79.Arai H, Miyamoto KI, Yoshida M, et al. The polymorphism in the caudal-related homeodomain protein Cdx-2 binding element in the human vitamin D receptor gene. J Bone Miner Res. 2001;16:1256–64. doi: 10.1359/jbmr.2001.16.7.1256. [DOI] [PubMed] [Google Scholar]
- 80.Smith EP, Boyd J, Frank GR, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–61. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 81.Korach KS. Insights from the study of animals lacking functional estrogen receptor. Science. 1994;266:1524–7. doi: 10.1126/science.7985022. [DOI] [PubMed] [Google Scholar]
- 82.Vidal O, Lindberg MK, Hollberg K, et al. Estrogen receptor specificity in the regulation of skeletal growth and maturation in male mice. Proc Natl Acad Sci USA. 2000;97:5474–9. doi: 10.1073/pnas.97.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Windahl SH, Vidal O, Andersson G, et al. Increased cortical bone mineral content but unchanged trabecular bone mineral density in female ERbeta(-/-) mice. J Clin Invest. 1999;104:895–901. doi: 10.1172/JCI6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ponglikitmongkol M, Green S, Chambon P. Genomic organization of the human oestrogen receptor gene. EMBO J. 1988;7:3385–8. doi: 10.1002/j.1460-2075.1988.tb03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kobayashi S, Inoue S, Hosoi T, et al. Association of bone mineral density with polymorphism of the estrogen receptor gene. J Bone Miner Res. 1996;11:306–11. doi: 10.1002/jbmr.5650110304. [DOI] [PubMed] [Google Scholar]
- 86.Albagha OM, McGuigan FE, Reid DM, et al. Estrogen receptor alpha gene polymorphisms and bone mineral density: haplotype analysis in women from the United Kingdom. J Bone Miner Res. 2001;16:128–34. doi: 10.1359/jbmr.2001.16.1.128. [DOI] [PubMed] [Google Scholar]
- 87.Ioannidis JP, Ralston SH, Bennett ST, et al. Differential genetic effects of ESR1 gene polymorphisms on osteoporosis outcomes. JAMA. 2004;292:2105–14. doi: 10.1001/jama.292.17.2105. [DOI] [PubMed] [Google Scholar]
- 88.Wang CL, Tang XY, Chen WQ, et al. Association of estrogen receptor alpha gene polymorphisms with bone mineral density in Chinese women: a meta-analysis. Osteoporos Int. 2007;18:295–305. doi: 10.1007/s00198-006-0239-2. [DOI] [PubMed] [Google Scholar]
- 89.Ioannidis JP, Stavrou I, Trikalinos TA, et al. Association of polymorphisms of the estrogen receptor alpha gene with bone mineral density and fracture risk in women: a meta-analysis. J Bone Miner Res. 2002;17:2048–60. doi: 10.1359/jbmr.2002.17.11.2048. [DOI] [PubMed] [Google Scholar]
- 90.Willing M, Sowers M, Aron D, et al. Bone mineral density and its change in white women: estrogen and vitamin D receptor genotypes and their interaction. J Bone Miner Res. 1998;13:695–705. doi: 10.1359/jbmr.1998.13.4.695. [DOI] [PubMed] [Google Scholar]
- 91.Patel MS, Cole DE, Smith JD, et al. Alleles of the estrogen receptor alpha-gene and an estrogen receptor cotranscriptional activator gene, amplified in breast cancer-1 (AIB1), are associated with quantitative calcaneal ultrasound. J Bone Miner Res. 2000;15:2231–9. doi: 10.1359/jbmr.2000.15.11.2231. [DOI] [PubMed] [Google Scholar]
- 92.Vandevyver C, Vanhoof J, Declerck K, et al. Lack of association between estrogen receptor genotypes and bone mineral density, fracture history, or muscle strength in elderly women. J Bone Miner Res. 1999;14:1576–82. doi: 10.1359/jbmr.1999.14.9.1576. [DOI] [PubMed] [Google Scholar]
- 93.Becherini L, Gennari L, Masi L, et al. Evidence of a linkage disequilibrium between polymorphisms in the human estrogen receptor alpha gene and their relationship to bone mass variation in postmenopausal Italian women. Hum Mol Genet. 2000;9:2043–50. doi: 10.1093/hmg/9.13.2043. [DOI] [PubMed] [Google Scholar]
- 94.Langdahl BL, Lokke E, Carstens M, et al. A TA repeat polymorphism in the estrogen receptor gene is associated with osteoporotic fractures but polymorphisms in the first exon and intron are not. J Bone Miner Res. 2000;15:2222–30. doi: 10.1359/jbmr.2000.15.11.2222. [DOI] [PubMed] [Google Scholar]
- 95.Brown MA, Haughton MA, Grant SF, et al. Genetic control of bone density and turnover: role of the collagen 1alpha1, estrogen receptor, and vitamin D receptor genes. J Bone Miner Res. 2001;16:758–64. doi: 10.1359/jbmr.2001.16.4.758. [DOI] [PubMed] [Google Scholar]
- 96.Han KO, Moon IG, Kang YS, et al. Nonassociation of estrogen receptor genotypes with bone mineral density and estrogen responsiveness to hormone replacement therapy in Korean postmenopausal women. J Clin Endocrinol Metab. 1997;82:991–5. doi: 10.1210/jcem.82.4.3879. [DOI] [PubMed] [Google Scholar]
- 97.Kobayashi N, Fujino T, Shirogane T, et al. Estrogen receptor alpha polymorphism as a genetic marker for bone loss, vertebral fractures and susceptibility to estrogen. Maturitas. 2002;41:193–201. doi: 10.1016/s0378-5122(01)00287-0. [DOI] [PubMed] [Google Scholar]
- 98.Ongphiphadhanakul B, Chanprasertyothin S, Payatikul P, et al. Oestrogen-receptor-alpha gene polymorphism affects response in bone mineral density to oestrogen in post-menopausal women. Clin Endocrinol. 2000;52:581–5. doi: 10.1046/j.1365-2265.2000.00979.x. [DOI] [PubMed] [Google Scholar]
- 99.Rapuri PB, Gallagher JC, Knezetic JA, et al. Estrogen receptor alpha gene polymorphisms are associated with changes in bone remodeling markers and treatment response to estrogen. Maturitas. 2006;53:371–9. doi: 10.1016/j.maturitas.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 100.Salmen T, Heikkinen AM, Mahonen A, et al. Early postmenopausal bone loss is associated with PvuII estrogen receptor gene polymorphism in Finnish women: effect of hormone replacement therapy. J Bone Miner Res. 2000;15:315–21. doi: 10.1359/jbmr.2000.15.2.315. [DOI] [PubMed] [Google Scholar]
- 101.Salmen T, Heikkinen AM, Mahonen A, et al. The protective effect of hormone-replacement therapy on fracture risk is modulated by estrogen receptor alpha genotype in early postmenopausal women. J Bone Miner Res. 2000;15:2479–86. doi: 10.1359/jbmr.2000.15.12.2479. [DOI] [PubMed] [Google Scholar]
- 102.Herrington DM, Howard TD, Brosnihan KB, et al. Common estrogen receptor polymorphism augments effects of hormone replacement therapy on E-selectin but not C-reactive protein. Circulation. 2002;105:1879–1882. doi: 10.1161/01.cir.0000016173.98826.88. [DOI] [PubMed] [Google Scholar]
- 103.Herrington DM, Howard TD, Hawkins GA, et al. Estrogen-receptor polymorphisms and effects of estrogen replacement on high-density lipoprotein cholesterol in women with coronary disease. N Engl J Med. 2002;346:967–74. doi: 10.1056/NEJMoa012952. [DOI] [PubMed] [Google Scholar]
- 104.Schuit SC, Oci HH, Witteman JC, et al. Estrogen receptor alpha gene polymorphisms and risk of myocardial infarction. JAMA. 2004;291:2969–2977. doi: 10.1001/jama.291.24.2969. [DOI] [PubMed] [Google Scholar]
- 105.Maruyama H, Toji H, Harrington CR, et al. Lack of an association of estrogen receptor alpha gene polymorphisms and transcriptional activity with Alzheimer disease. Arch Neurol. 2000;57:236–40. doi: 10.1001/archneur.57.2.236. [DOI] [PubMed] [Google Scholar]
- 106.Morton MS, Arisaka O, Miyake N, et al. Phytoestrogen concentrations in serum from Japanese men and women over forty years of age. J Nutr. 2002;132:3168–71. doi: 10.1093/jn/131.10.3168. [DOI] [PubMed] [Google Scholar]
- 107.Uehar M, Arai Y, Watanabe S, et al. Comparison of plasma and urinary phytoestrogens in Japanese and Finnish women by time-resolved fluoroimmunoassay. Biofactors. 2000;12:217–25. doi: 10.1002/biof.5520120134. [DOI] [PubMed] [Google Scholar]
- 108.Kim MK, Chung BC, Yu VY, et al. Relationships of urinary phytooestrogen excretion to BMD in postmenopausal women. Clin Endocrinol. 2002;56:321–8. doi: 10.1046/j.1365-2265.2002.01470.x. [DOI] [PubMed] [Google Scholar]
- 109.Yim CH, Choi JT, Choi HA, et al. Association of estrogen receptor alpha gene microsatellite polymorphism with annual changes in bone mineral density in Korean women with hormone replacement therapy. J Bone Miner Metab. 2005;23:395–400. doi: 10.1007/s00774-005-0619-2. [DOI] [PubMed] [Google Scholar]
- 110.Westberg L, Baghaei F, Rosmond R, et al. Polymorphisms of the androgen receptor gene and the estrogen receptor beta gene are associated with androgen levels in women. J Clin Endocrinol Metab. 2001;86:2562–8. doi: 10.1210/jcem.86.6.7614. [DOI] [PubMed] [Google Scholar]
- 111.Chen HY, Chen WC, Tsai HD, et al. Relation of the estrogen receptor alpha gene microsatellite polymorphism to bone mineral density and the susceptibility to osteoporosis in postmenopausal Chinese women in Taiwan. Maturitas. 2001;40:143–50. doi: 10.1016/s0378-5122(01)00233-x. [DOI] [PubMed] [Google Scholar]
- 112.Chen WC, Wu HC, Lin WC, et al. The association of androgen-and oestrogen-receptor gene polymorphisms with urolithiasis in men. BJU Int. 2001;88:432–6. doi: 10.1046/j.1464-410x.2001.02319.x. [DOI] [PubMed] [Google Scholar]
- 113.Sano M, Inoue S, Hosoi T, et al. Association of estrogen receptor dinucleotide repeat polymorphism with osteoporosis. Biochem Biophys Res Commun. 1995;217:378–83. doi: 10.1006/bbrc.1995.2787. [DOI] [PubMed] [Google Scholar]
- 114.Sowers M, Willing M, Burns T, et al. Genetic markers, bone mineral density, and serum osteocalcin levels. J Bone Miner Res. 1999;14:1411–9. doi: 10.1359/jbmr.1999.14.8.1411. [DOI] [PubMed] [Google Scholar]
- 115.Ongphiphadhanakul B, Chanprasertyothin S, Payattikul P, et al. Association of a T262C transition in exon 1 of estrogen-receptoralpha gene with skeletal responsiveness to estrogen in postmenopausal women. J Endocrinol Invest. 2001;24:749–55. doi: 10.1007/BF03343923. [DOI] [PubMed] [Google Scholar]
- 116.Kozak M. Initiation of translation in prokaryotes and eukaryotes. Gene. 1999;234:187–208. doi: 10.1016/s0378-1119(99)00210-3. [DOI] [PubMed] [Google Scholar]
- 117.Afshar-Kharghan V, Li CQ, Khoshnevis-Asl M, et al. Kozak sequence polymorphism of the glycoprotein (GP) Ibalpha gene is a major determinant of the plasma membrane levels of the platelet GP Ib-IX-V complex. Blood. 1999;94:186–91. [PubMed] [Google Scholar]
- 118.Vesell ES. Pharmacogenetics perspectives gained from twin and family studies. Pharmacol Ther. 1989;41:535–52. doi: 10.1016/0163-7258(89)90130-7. [DOI] [PubMed] [Google Scholar]
- 119.Kalow W, Tang BK, Endrenyi I. Hypothesis: comparisons of inter-and intra-individual variations can substitute for twin studies in drug research. Pharmacogenetics. 1998;8:283–9. doi: 10.1097/00008571-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 120.Evans WE, McLeod HL. Pharmacogenomics--drug disposition, drug targets, and side effects. N Engl J Med. 2003;348:538–49. doi: 10.1056/NEJMra020526. [DOI] [PubMed] [Google Scholar]
- 121.Kalow W. Familial incidence of low pseudocholinesterase level. Lancet. 1956;2:576. doi: 10.1016/s0140-6736(56)90869-8. [DOI] [PubMed] [Google Scholar]
- 122.Carson PE, Flanagan CI, Ickes CE, et al. Enzymetic deficiency in primaquine-sensitive erythrocytes. Science. 1956;124:484–5. doi: 10.1126/science.124.3220.484-a. [DOI] [PubMed] [Google Scholar]