Abstract
Immunological tolerance is one of the fundamental aspects of the immune system. The CD4+CD25+ regulatory T (Treg) cells have emerged as key players in the development of tolerance to self and foreign antigens. However, little is known about the endogenous factors and mechanisms controlling their suppressive capacity on immune response. In this study, we observed that docosahexaenoic acid (DHA), an n-3 polyunsaturated fatty acid, diminished, in a dose-dependent manner, the capacity of Treg cells to inhibit the CD4+CD25− effector T-cell proliferation. DHA not only reduced the migration of Treg cells toward chemokines but also downregulated the mRNA expression of CCR-4 and CXCR-4 in Treg cells. DHA also curtailed ERK1/2 and Akt phosphorylation and downregulated the Smad7 levels in these cells. Contradictorily, DHA upregulated the mRNA expression of Foxp3, CTLA-4, TGF-β, and IL-10; nonetheless, this fatty acid increased the expression of p27KIP1 mRNA, known to be involved in Treg cell unresponsiveness. In Foxp3-immunoprepitated nuclear proteins, DHA upregulated histone desacetylase 7 levels that would again participate in the unresposnsiveness of these cells. Finally, a DHA-enriched diet also diminished, ex vivo, the suppressive capacity of Treg cells. Altogether, these results suggest that DHA, by diminishing Treg cell functions, may play a key role in health and disease.
Keywords: extracellular signal-regulated kinase 1/2, Smad7, histone desacetylase 7
CD4+CD25+ regulatory T (Treg) cells were initially defined as a subpopulation of suppressor T-cells that mediate immune tolerance by suppressing autoreactive CD4+ or CD8+ T-cells (1, 2). They represent 1–3% of total CD4+ T-cells in human and 5–10% in rodents and express cell surface molecules associated with activated/memory T-cells [CD25, CD45Rblow, GITR, cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), and CD103], as well as the forkhead/winged helix transcription factor protein-3 (Foxp3) (3, 4). Several types of Treg cells have been identified and characterized as natural and adaptive or inducible Treg cells (iTreg). The cells referred to as natural Treg cells that originate in the thymus are self-reactive (5) and possess the ability to suppress the activation of T effector (Teff) cells in a cell-contact-dependent, interleukin-10 (IL-10)-independent, and transforming growth factor (TGF-β)-independent manner (1, 2). However, the iTregs arise in the periphery, either during an immune response or after encountering tolerogenic dendritic cells. These cells are believed to differentiate from naïve precursors and are specific for antigens not presented in the thymus, such as food antigens, bacterial flora, pathogens, and self-antigens such as insulin (6). They suppress the activation of effector T-cells in a cytokine-dependent manner: TGF-β for Th3 cells and IL-10 for T regulatory 1 cells (7).
One crucial question is whether Treg cells can be induced (iTregs) or converted from normal peripheral CD4+ T-cells, and if this occurs, which are the molecules and/or cytokines responsible for this transition, since activated CD4+ T-cells, expressing CD25 under neutral TCR stimulation conditions show no suppressive ability (1, 2). Some investigators have shown that prostaglandin E2 (PGE2) induced a regulatory phenotype and conferred T regulatory activity in CD4+CD25− effector T-cells (8). These authors have also shown that PGE2 enhanced the in vitro inhibitory function of human Treg cells. On the other hand, some investigators have demonstrated that dopamine, through the extracellular signal-regulated kinase pathway, reduced the suppressive activity and the adhesive and migratory abilities of Treg cells (9).
Several studies have recently shown that n-3 PUFA, abundantly present in fish oils, inhibit cancer cell growth (10, 11). Besides, n-3 PUFA exert immunosuppressive effects on T-cell proliferation (12–14). Animal studies also suggest that the diets rich in n-3 PUFA, containing eicosapentaenic acid (EPA) and docosahexenoic acid (DHA), are anti-inflammatory and immunomodulatory (13, 14). Since Treg cells modulate inflammation and cancer cell growth, it was thought worthwhile to investigate the effects of DHA on Treg cell functions. All agents capable of reducing Treg suppressive activity, or their trafficking ability, or both, might therefore be promising candidates for therapy against cancer as, in this pathology, the decreased Treg cell functions are responsible for the upregulated abilities of T effector cells to eliminate cancer cells. In fact, increased Treg cell activity has been shown to be correlated to CD8+ T-cell impairment and, therefore, poor survival of cancer patients (15). Circulating CD4+CD25+ Treg numbers correlate with tumor stage in patients with gastric and esophageal cancers (16). In addition, increased Treg was found to be associated with high mortality in ovarian carcinoma patients, suggesting that these cells may play a generalized role in disease progression (17).
MATERIALS AND METHODS
Animals
The study was performed on male animals, wild-type C57BL6/J mice, purchased from Charles River (Les Oncins, France). For acclimatization, mice were housed in wood-chip-bedded plastic cages at constant temperature (25°C) and humidity (60 ± 5%) with a 12 h light-dark cycle. The general guidelines for the care and use of laboratory animals, recommended by the council of European Economic Communities, were followed. The experimental protocol was approved by the Regional Ethical Committee.
Cell isolation and culture
The removed spleen was immediately transferred to the Petri dishes containing RPMI 1640 medium (BioWhittaker, Liège, Belgium) supplemented with the following: 25 mM HEPES, 2 mM l-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin. For the cell isolation, the spleen was teased apart using a wire gauge. The number of viable cells was determined using the trypan blue exclusion test. After centrifugation, the cells were resuspended in the complete RPMI 1640 medium supplemented with 10% FBS, and the total splenic T-cells were isolated using the Nycoprep 1.077A solution (ABCyS, Paris, France). After washing, CD4+CD25− T-cells and Treg cells were then isolated using the Treg isolation kit (Miltenyi Biotec, CA) and the Magnetic cell sorting column (Miltenyi Biotec). Cell purity assessed by flow cytometry was ≥90% (data not shown). Before being used in different experimental conditions, purified CD4+CD25+ (Treg) and CD4+CD25− (Teff) cells were preincubated or not for 4 h with or without three different concentrations of DHA in the complete RPMI 1640 medium containing 10% FBS and 0.2% BSA. The 4 h incubation period with DHA was chosen because this was the sufficient time for this fatty acid for its incorporation into the cell plasma membranes. The inhibitory effect of Treg cells on Teff cell proliferation was performed by culturing purified CD4+CD25− T-cells as responders in the presence of purified CD4+CD25+ Treg cells.
In vitro cell proliferation assay
The proliferation assay was performed in the presence of the autologous accessory cells obtained during T-cell purification. We treated the accessory cells with mitomycin C (50 μg/ml) in order to inhibit the proliferation of these cells. Before starting the proliferation assay, DHA was removed by washing the cells in complete RPMI 1640 medium containing 0.5% BSA. DHA-treated or -untreated Teff cells were then cultured at a 5:1 ratio with autologous DHA-treated or -untreated Treg cells in the presence of soluble anti-CD3 antibody (1 µg/well) in 96-well flat-bottom tissue culture plates (BD Bioscience, Franklin Lakes, NJ) for 46 h. This ratio (5:1) was found to be optimal for the ability of Treg cells to suppress the responder T (Teff) cell proliferation (data not shown). Cells were distributed in six replicates as follows: 180 µL of cell suspension and 20 µL of mitogen or complete RPMI 1640 medium.
Plates were incubated for 46 h at 37°C in a 5% CO2/air atmosphere. For the last 6 h, 0.8 µCi/well of [3H]thymidine in 20 µL of complete RPMI medium was added. At the end of the incubation, cells were collected using a cell harvester (Dynatec, Vienna, Austria), trapping their DNA onto glass filter mats. When the filter circles were dried, we placed them in plastic minivials (Packard, Paris, France) and added with 4 ml Optifluor-O (Packard). The radioactivity was recorded in a scintillation counter (Packard). Results are expressed as the percentage of proliferation of responder T-cells (Teff).
We also employed carboxyfluorescien succinimidyl ester (CFSE; Molecular Probes), at 2 μM, in some experiments to be sure that we were only altering the Teff cell population since Treg cells were anergic, particularly to show that DHA-treated Treg cells do lose their capacity to inhibit Teff cell proliferation. We labeled the CD4+ T-cells with CFSE and assessed their proliferation (46 h proliferation assay) with DHA-treated Treg cells. Hence, coculture experiments by means of CFSE and [3H]thymidine incorporation assays gave similar results (data not shown). We also employed trichostatin A (TSA; Calbiocem, France), an inhibitor of histone desacetylase (HDAC), in Teff cell proliferation assays.
Real-time RT-PCR quantification assay
Total RNA was prepared from the DHA-treated and untreated purified CD4+CD25+ and CD4+CD25− T-cells using Trizol reagent (Invitrogen Life Technologies, Groningen, The Netherlands) according to the manufacturer's instructions. The integrity of RNA was electrophoretically checked by ethidium bromide staining and by optical density (OD) absorption ratio OD260nm/OD280nm > 1.9. One microgram of total RNA was reverse transcribed with Super script II RNase H-reverse transcriptase using oligo(dT) according to the manufacturer's instructions (Invitrogen Life Technologies).
Real-time PCR was performed on iCycler iQ real-time detection system (Bio-Rad), and amplification was done using SYBR® Green I detection (SYBR® Green JumpStartTM, Taq ReadyMixTM for quantitative PCR; Sigma-Aldrich). Oligonucleotide primers, used for mRNA analysis, were based on the sequences of mice genes in the GenBank database. The sequences of the primers set used to amplify the genes in these studies are presented in Table 1.
TABLE 1.
Amplified mouse genes and their corresponding primer sequences used for real-time quantitative PCR
| Genes Amplified | Primer Sequences |
|---|---|
| Mouse β-actin | forward: 5′-GGCACCACACCTTCTACAATGAGC-3′ |
| reverse: 5′-CGACCAGAGGCATACAGGGACAG-3′ | |
| Mouse Foxp3 | forward: 5′-CCTATGGCTCCTTCCTTGGC-3′ |
| reverse: 5′-ATGAAGTGTGGTCTGTCCTGG-3′; | |
| Mouse CTLA-4 | forward: 5′-TGGGTTCAAACACATCTCAAGGC-3′ |
| reverse: 5′-TGTCGTGGCACAGACCTCAG-3′ | |
| Mouse IL-10 | forward: 5′-GGTTGCCAAGCCTTATCGGA-3′ |
| reverse: 5′-ACCTGCTCCACTGCCTTGCT-3′ | |
| Mouse TGF-β | forward: 5′-TCTCCCTCAACCTCAAATTATTC-3′ |
| reverse: 5′-GAGCAGAAGCGGCAGTAG-3′ | |
| Mouse CCR-4 | forward: 5′-CTGTTGTGGTTCTGGTCCTGTTC-3′ |
| reverse: 5′-CGTGTGGTTGTGCTCTGTGTAG-3′ | |
| Mouse CCR-8 | forward: 5′-TGCGATGTGTAAGGTGGTCTCTGG-3′ |
| reverse: 5′-TGATGGCTCTGGTCCTGTTGTGG-3′ | |
| Mouse CXCR-4 | forward: 5′-CCTCTACAGCAGCGTTCTCATCC-3′ |
| reverse: 5′-CACCACCATCCACAGGCTATCG-3′ | |
| Mouse p27Kip1 | forward: 5′-CGGCGGCAAGGTTTGGAGAGG-3′ |
| reverse: 5′-GGAGGAGGCAGGAGGAGGTGG-3′ | |
| Mouse L-selectin | forward: 5′-CTGTGATGCAGGGTATTACGGG-3′ |
| reverse: 5′-CTCTCTTCCCTCAGAACAGTTG-3′ |
The amplification was carried out in a total volume of 25 µl containing 12.5 µl SYBR® Green Taq ReadyMixTM, 0.3 µM each primer, and diluted cDNA. The standard curves were generated for each protein or β-actin using serial dilutions of positive control template to establish PCR efficiencies.
Western blotting
Akt and mitogen-activated protein kinase, extracellular signal-regulated kinase (ERK1/2), expression was analyzed in the lysates of Treg and Teff (2.0 × 106) cells preincubated for 1 h with or without DHA or U0126, a mitogen-activated protein kinase inhibitor (Ozyme, Saint Quentin, France). After incubation, the cells were stimulated with anti-CD3 (2 µg/ml) and anti-CD28 (5 µg/ml) antibodies for 20 min. For Smad7, acetylated lysine resides, or HDAC7, the cells were preincubated with DHA for 4 h and then stimulated with anti-CD3 (2 µg/ml) and anti-CD28 (5 µg/ml) antibodies overnight. The cells were washed, and the protein concentration in the cell lysates was determined using a bicinchoninic acid assay (Pierce, France). Protein-normalized aliquots of the cell lysate (20 µg) were separated by electrophoresis on a 10% SDS-PAGE and transferred onto nitrocellulose membranes. The protein was immunodetected with 1/2,000 anti-mouse phospho-ERK1/ERK2 or anti-mouse pospho-Akt monoclonal antibodies (Ozyme). The blots were developed with 1/2,000 HRP-conjugated anti-rabbit Ig (Santa Cruz Biotechnology) and the ECL detection system (Amersham, France), followed by autoradiography. Relative protein quantification was determined by computerized densitometric analysis using Scion Image software (version 1.62c).
For the detection of acetylated lysine residues and HDAC7, the nuclear fractions were prepared essentially as described by Dignam et al. (18) with some modifications. After treatment, cells (20 × 106) were washed with PBS without calcium and magnesium salts by centrifugation (250 g, 10 min) at room temperature. Cell pellets were resuspended in 5 volumes of ice-cold cell homogenization buffer (10 mM HEPES-KOH, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, 0.5 mM PMSF, and 2 µl/ml protease inhibitor cocktail), left on ice for 10 min, and then centrifuged (250 g, 10 min) at room temperature. The cell pellets were suspended in 3 volumes of ice-cold cell homogenization buffer containing 0.05% (v/v) Nonidet P-40, and then cells were lysed with 20 strokes of a tight-fitting Dounce homogenizer. Nuclei were collected by centrifugation (250 g, 10 min) at 4°C. Pellets of nuclei were resuspended first in 300 µl of hypotonic buffer (40 mM HEPES-KOH, pH 7.9, 0.4 M KCl, 1 mM DTT, 0.1 mM PMSF, 10% glycerol, and 2 µl/ml protease inhibitor cocktail), and then NaCl was added to a final concentration of 300 mM. The mixture was left at 4°C for 30 min. After centrifugation (100,000 g, 20 min), the supernatant was divided into aliquots of 50 µl and stored at –80°C.
We immunoprecipitated the nuclear fractions with anti-Foxp3 antibodies and then detected with antibodies against HDAC7 (sc-11489; Santa Cruz Biotechnology) and acetylated lysine (sc-81623; Santa Cruz Biotechnology). Briefly, 1 mg of protein was immunoprecipitated with 1 µg of anti-Foxp3 antibody and 25 µl of A/G-Sepharose beads. The immunoprecipitates were washed three times with buffer A and diluted with Laemmli sample buffer. For Western blotting, denatured proteins (20 µg) were separated on SDS-polyacrylamide/Bis acrylamide (8%) gel and transferred onto the nitrocellulose membranes as described above.
For the detection of p27Kip1 protein, Treg or Teff cells were incubated without or with DHA (100 µM) for 4 h and then used for Western blot detection by employing anti-p27Kip1 (Santa Cruz Biotechnology) and peroxidase-conjugated secondary antibodies (goat anti-rabbit) as described above.
Chemotaxis assays
The migration of Treg and Teff cells across polycarbonate filters (pore size, 5 µm; diameter, 6.5 mm) toward stroma cell-derived factor-1 (SDF-1) and macrophage-derived chemokine (MDC/CCL22) (PeproTech, Levallois Perret, France) was assayed in 24-well Transwell chambers (Costar Corning, Corning, NY). Purified Treg or Teff cells (1.67 × 106 cells/ml) were suspended in RPMI 1640 complete medium, and 150 µL of the cell suspension was added to the upper chamber (9) after incubation with or without DHA (100 µM, 4 h, 37°C, 5% CO2). Chemokines were added to the lower chamber at concentrations of 1 µg/ml SDF-1 and 0.25 µg/ml MDC (9). The plates were incubated for 12 h at 37°C in 5% CO2. The numbers of migrating cells were determined by counting under the microscope. The number of Teff or Treg cells that migrated was expressed as a percentage of the total cell population before migration. L-selectin mRNA expression was also analyzed in cells (Teff or Treg), pretreated or not with DHA (100 µM, 4 h, 37°C, 5% CO2).
Feeding the animals a DHA-enriched diet for ex vivo assays
To investigate the effect of dietary DHA, we constituted two diet groups of animals. The first group of 10 mice was fed a standard diet containing vegetable oil (ISIO-4 oil), and the other group of 10 mice was fed a DHA diet (containing EPA and DHA) for 6 weeks before cell isolation and culture.
The chemical composition of the standard diet was as follows (g/kg dry diet): starch, 587; casein, 200; cellulose, 50; sucrose, 50; mineral mix, 40; vitamin mix, 20; dl-methionine, 3; vegetable oil Isio-4 (Lesieur, Neuilly-sur-Seine, France), 50. The Isio-4 oil contained in mg/g: 47.2, linoleic acid (18:2n-6), 1.7, total (n-3) and MUFAs 40.2 (largely 18:1). Total oil represented 5% of the diet (16–19). In the DHA diet, half of the Isio-4 oil was replaced by EPAX oil (EPAX-7010). The EPAX oil, in the form of ethyl ester, contained ∼85% n-3 PUFA (i.e., 70% EPA, 12% DHA, and from 2.1–3.2% α-tocopherol). It means that EPAX oil represented 2.5% of the DHA-diet. The composition of the mineral and the vitamin mix was identical as described by Triboulot et al. (19). EPAX oil was tightly sealed under a stream of nitrogen to avoid lipid oxidation and kept at 4°C. Diets were prepared every day, and the mice consumed them ad libitum. Uneaten food was discarded; food cups and water bottles were washed frequently.
Ex vivo proliferation assays and mRNA expression
After 6 weeks of diet feeding, the mice were anesthetized and the spleen was removed. The Treg and Teff cells were isolated from the removed spleen as described above for ex vivo proliferation assay. The inhibitory effect of Treg cells on Teff cell proliferation was performed as described above. The cells were also removed to analyze the mRNA expression of Foxp3, CTLA-4, TGF-β, and IL-10 by real-time quantitative RT-PCR.
Lipid determination in the diets and cell plasma membrane
The details of the fatty acid composition of the standard diet and the DHA diet appear in the Table 2. Total lipids were extracted from the diets according to the method of Bligh and Dyer (20) then transmethylated by BF3/methanol after saponification, and fatty acids were analyzed by gas liquid chromatography (21) using C17:0 as internal standard with a Becker gas chromatograph (Becker Instruments, Downers Grove, IL) equipped with a 50 m capillary glass column packed with carbowax 20 m (Spiral-RD, Couternon, France).
TABLE 2.
Fatty acid composition of standard and DHA-enriched diets
| Fatty Acids | Standard Diet (mg/g) | DHA Diet (mg/g) |
|---|---|---|
| C14:0 | 0.4 | 0.4 |
| C16:0 | 5.1 | 2.1 |
| C18:0 | 3.9 | 1.7 |
| C18:1 | 18.5 | 9.1 |
| C18:2n-6 | 21.3 | 11.2 |
| C18:3n-3 | 0.83 | 0.5 |
| C20:4n-6 (AA) | ND | 0.9 |
| C20:5n-3 (EPA) | ND | 22.2 |
| C22:6n-3 (DHA) | ND | 2.0 |
| Total FA | 50.0 | 50.0 |
| ∑ n-6 PUFA | 21.30 | 12.06 |
| ∑ n-3 PUFA | 0.83 | 24.59 |
| (n-6)/(n-3) | 25.80 | 0.49 |
| (n-3)/(n-6) | 0.04 | 2.04 |
| ∑ SFA | 9.40 | 4.26 |
| ∑ PUFA | 22.13 | 36.65 |
| ∑ MUFA | 18.50 | 9.07 |
| PUFA/SFA | 2.35 | 8.60 |
ND, not detectable.
Fatty acid analysis of the phospholipids of cell plasma membrane
The lipids from Treg and Teff cells were extracted according to the method of Bligh and Dyer (20). Phospholipids were separated on silica gel by TLC using the following solvent: chloroform/methanol/acetic acid at 35:14:2.7 (v/v/v). After scraping off, the phospholipid fractions were transmethylated by BF3/methanol after saponification, and fatty acids were extracted and further analyzed by gas liquid chromatography (21). Analysis of fatty acid peaks was achieved with reference to the internal standard by using DELSI ENICA 21 integrator (Delsi Nermag, Rungis, France).
Statistical analysis
Results are shown as means ± SEM. The significance of the differences between mean values was determined by two-way ANOVA (STATISTICA, version 4.1; Statsoft, Paris, France), followed by the least significant difference test. Differences were considered significant at P < 0.05.
RESULTS
DHA curtails the suppressive capacity of Treg cells on Teff cell proliferation
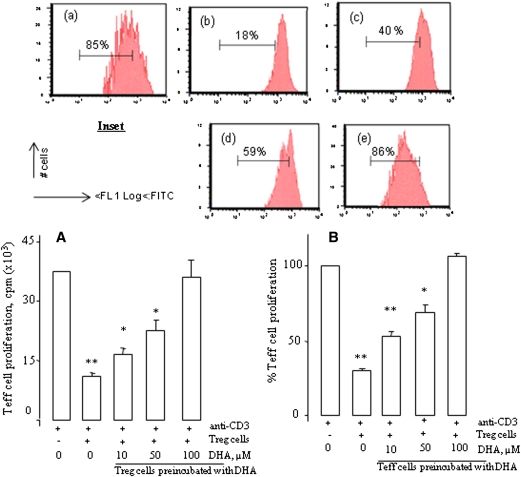
Coculturing of CD4+CD25− Teff and CD4+CD25+ Treg cells results in the suppression of Teff cell proliferation (Fig. 1). In order to examine the effects of DHA on the suppressive capacity of Treg cells on Teff cell proliferation in vitro, Treg cells were preincubated with increasing concentrations of DHA (Fig. 1A). We observed that DHA decreased the in vitro inhibitory functions of Treg cells on Teff cell proliferation in a dose-dependent manner (Fig. 1A). DHA completely reversed the inhibitory effect of Treg cells at 100 µM. In CFSE-labeled Teff cells that were cocultured with DHA-treated Treg cells, we observed the same results (Fig. 1, inset).
Fig. 1.
DHA reduces Treg cell functions. A: CD4+CD25− T (Teff) cells as responder cells and autologous CD4+CD25+ regulatory T (Treg) cells, both purified from the spleen of mice, were cocultured with soluble anti-CD3/CD28 antibody for 46 h. Treg cells were preincubated or not with increasing concentrations of DHA for 4 h. After incubation, the cells were washed and used for the 46 h proliferation assay. B: The experimental protocol was the same as in A except that Teff cells were preincubated with increasing concentrations of DHA for 4 h. After incubation, the cells were washed and used for the 46 h proliferation assay. In all experiments, cell proliferation was measured by [3H]thymidine incorporation. Results are expressed as mean ± SEM of experiments reproduced at least three times and performed in six replicates from different mice. *P < 0.05 and **P < 0.01: significant difference compared with control cells. Inset shows the Treg suppression assays in which Teff cells were labeled with CFSE (2 µM) and stimulated as described above. In the cell, esterases cleave the acetyl group, leading to the fluorescent diacetylated CFSE. The percentages of residual proliferating cells are indicated in the inset figures. The treatments were as follows: (a), Control Teff cells; (b), Treg cells + Treg cells; (c), Treg cells + Teff cells (preincubated with DHA at 10 μM); (d), Treg cells + Teff cells (preincubated with DHA at 50 μM); (e), Treg cells + Teff cells (preincubated with DHA at 100 μM). Data are representative of three separate experiments.
In order to assess whether the DHA-pretreated responder (Teff) cells will act differently in the presence of Treg cells, we further conducted our experiments by preincubating the Teff cells with increasing concentrations of DHA. Once again, we observed that the suppressive activity of Treg cells on DHA-treated Teff cell proliferation was diminished, in a dose-dependent manner (Fig. 1B). Similarly, the inhibitory effect of Treg cells was completely diminished by DHA at 100 µM. By keeping in view these observations, we conducted our further experiments at a concentration of 100 µM of DHA.
DHA upregulates Foxp3, CTLA-4, and TGF- β mRNA expression in Treg cells
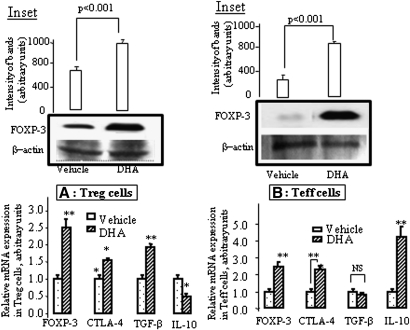
Since PGE2, one of the arachidonic acid (AA; 20:4n-6) metabolites, induced the expression of Foxp3 mRNA in Teff cells and enhanced its expression in Treg cells (8), we examined the effect of DHA on the mRNA expression of the major markers reported to be characteristics of Treg cells (22). Both Teff and Treg cells were separately incubated with DHA (100 µM). We observed that this fatty acid increased the expression of Foxp3, CTLA-4, and TGF-β mRNA and decreased that of IL-10 in Treg cells (Fig. 2A). In Teff cells, a treatment by DHA induced the expression of Foxp3, CTLA-4, and IL-10, but not of TGF-β mRNA (Fig. 2B). DHA also upregulated the expression of Foxp3 protein levels in both the cells (Fig. 2A, B, insets).
Fig. 2.
DHA induces Foxp3, CTLA-4, TGF-β, and/or IL-10 mRNA expression. The cells were purified from the spleen of mice and preincubated without or with DHA (100 µM) for 4 h, prior to the total RNA extraction from Treg (A) and Teff cells (B). The mRNA expression of the genes was quantitatively determined by real-time PCR as described in Materials and Methods. Insets of A and B show the expression of Foxp3 protein in Treg and Teff cells, respectively, treated or not with DHA (100 μM).
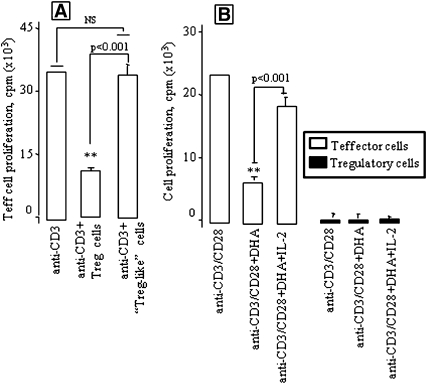
DHA does not confer the Treg-like suppressive activity to Teff cells
Since DHA induced the Treg-like phenotype in Teff cells by upregulating the expression of mRNA of Treg cells (as mentioned here above), we were interested in analyzing whether DHA-treated Teff cells (so-called Treg-like) can suppress responder T (Teff) cell proliferation. Hence, we cocultured normal CD4+CD25− Teff cells in the presence of DHA-treated Teff (Treg-like) cells. We noticed that the latter failed to suppress the proliferation of the former (Fig. 3A), indicating that the so called Treg like cells did not acquire Treg suppressive activity, even though DHA induced Treg-like phenotype in these cells. Furthermore, we observed that DHA inhibited the proliferation of Teff cells, which was reversed by exogenous IL-2 (Fig. 3B). However, IL-2 failed to influence the proliferation of DHA-treated Treg cells, though high concentration of IL-2 (500 U/ml) in the absence of DHA stimulated the proliferation of Treg cells (data not shown) as described elsewhere (23).
Fig. 3.
DHA-treated Treg-like cells do not behave as true Treg cells. Teff cells as responder cells and autologous Treg-like cells (DHA-treated Teff cells, DHA at 100 µM) or Treg cells were cocultured with soluble anti-CD3 antibody for 46 h (A). Treg or Teff cells, containing or not DHA (100 μM), were stimulated with anti-CD3 (2 µg/ml) and anti-CD28 (5 µg/ml) antibodies for 46 h with or without IL-2 at 20 IU/ml (B). Cell proliferation was measured by [3H]thymidine incorporation. Results are expressed as mean ± SEM of experiments reproduced at least three times and performed in six replicates from different mice. **P < 0.001: significant difference compared with controls. NS, insignificant differences.
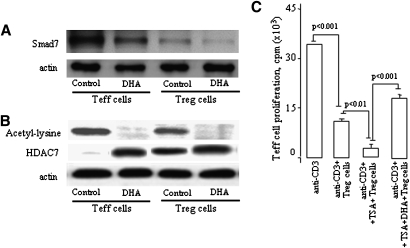
DHA modulates Smad7 and HDAC7 levels, and HDAC inhibitor upregulates the Treg activity
Fantini et al. (24) have recently shown that the inhibitory Smad7 protein that is normally induced by TGF-β is strongly downregulated by Foxp3 at the transcriptional level. Hence, the downregulation of Smad7 was found associated to upregulated expression of Foxp3 in CD4+CD25− T cells. We observed that DHA downregulated the Smad7 levels both in Treg and Teff cells (Fig. 4A).
Fig. 4.
DHA modulates Smad7 and HDAC7 levels, and HDAC inhibitor upregulates Treg activity. Treg and Teff (2.0 × 106) cells preincubated with or without DHA for 4 h. After incubation, the cells were stimulated with anti-CD3 (2 µg/ml) and anti-CD28 (5 µg/ml) antibodies for 24 h. Smad7 detection was performed on whole-cell lysates (A); however, for the detection of acetylated lysine residues and HDAC7 (B), the nuclear fractions were prepared and immunoprecipitated with anti-Foxp3 antibodies and subjected to immunodetection with antibodies against HDAC7 and acetylated lysine as described in Materials and Methods. C: The Teff cell proliferation assays were performed as described in the legends of Figure 1 except the Treg cells were pretreated or not with TSA (400 nM) for 4 h and were further incubated or not with DHA (at 100 μM) for 4 h and after washing were used for the assays.
The remodeling of chromatin is believed to be a critical component of transcriptional regulation, and a major source of this remodeling is brought about by the acetylation of nucleosomal histones. Acetylation of lysine residues in the N-terminal tail domain of histones results in an allosteric change in the nucleosomal conformation and an increased accessibility to transcription factors by DNA. Conversely, the deacetylation of histones is associated with transcriptional silencing. Mammalian HDAC7 is a histone deacetylase that interacts with the adaptor mSin3A. The interaction of HDAC7 with mSin3A suggests the association of multiple repression complexes of transcription factors. Whether deacetylation is playing a role in Treg cell functions, we studied the expression of HDAC7 and acetylated histones in Treg and Teff cells. We observed that Treg cells expressed both HDAC7 and acetylated histones (Fig. 4B). Furthermore, DHA induced HDAC7 expression only in Teff cells, though this fatty acid diminished the levels of acetylated histones in Treg and Teff cells (Fig. 4B).
Fig. 4C shows the effects of TSA, an inhibitor of HDAC activity. We observed that TSA significantly upregulated the Treg suppressive capacity. Interestingly, DHA curtailed the TSA-upregulated capacity of Treg cells, though this fatty acid failed to completely abolish the suppressive activity of Treg cells.
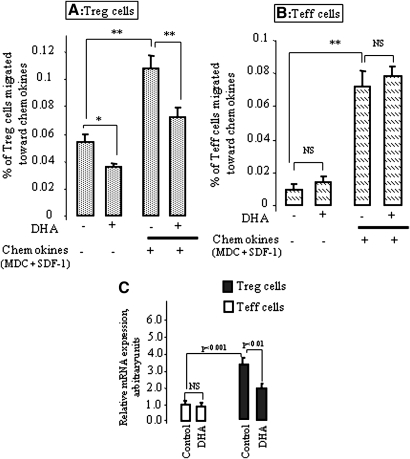
DHA alters migratory properties and L-selectin mRNA expression of Treg cells
Migration of Treg cells in humans is dependent on the chemokine receptors, e.g., CCR-4, CCR-8, and CXCR-4, which are abundantly present on these cells (25). Therefore, we examined whether DHA exposure would affect Treg and Teff cell migration toward CCL22 (MDC, a chemokine for CCR-4) and CXCL12 (SDF-1, a chemokine for CXCR-4). Our results showed that the chemokines induced a significant increase of Treg and Teff cell migration (Fig. 5A, B). Exposure of Treg cells to DHA significantly reduced their migration across the polycarbonate membrane in the presence or absence of chemokines (Fig. 5A). However, DHA failed to decrease the migration of Teff cells in the presence or absence of chemokines (Fig. 5B).
Fig. 5.
DHA alters the migratory properties and L-selectin mRNA expression of Treg, but not of Teff, cells. Purified Treg (A) and Teff (B) cells were incubated without or with DHA (100 µM) for 4 h and then subjected to the migration assay toward the chemokines (MDC plus SDF-1) as described in Materials and Methods. The L-selectin mRNA expression was quantitatively determined by real-time PCR (C) in DHA-treated (100 µM) or untreated Treg and Teff cells as described in Materials and Methods. *P < 0.05 and **P < 0.001: significant difference compared with corresponding control. NS, nonsignificant differences.
We also assessed the expression of L-selectin mRNA expression in Treg and Teff cells. We observed that DHA inhibited the expression of L-selectin in Treg cells (Fig. 5C). However, this fatty acid failed to influence the expression of the same in Teff cells that had a lower level of L-selectin mRNA expression than Treg cells (Fig. 5D).
DHA modulates the mRNA expression of the chemokine receptors in Treg and Teff cells
In an attempt to link the changes in the migration to specific receptors, we examined the mRNA expression of CCR-4, CCR-8, and CXCR-4 in Treg and Teff cells. First, we observed that, under no treatment, Treg cells expressed higher CCR-4, CCR-8, and CXCR-4 mRNA than Teff cells (Table 3). However, the exposure to DHA significantly reduced CCR-4 and CXCR-4 mRNA in Treg, but not in Teff, cells (Table 3).
TABLE 3.
Relative mRNA expression (arbitrary units) of the chemokine receptors in Treg and Teff cells
| Treg Cells |
Teff Cells |
|||
|---|---|---|---|---|
| DHA (100 µM) | Vehicle | DHA (100 µM) | Vehicle | |
| CCR-4 | 1.55 ± 0.19 | 3.01 ± 0.2†* | 0.93 ± 0.07 | 1.0 ± 0.1† |
| CCR-8 | 2.90 ± 0.2 | 2.92 ± 0.18 | 0.93 ± 0.09 | 1.0 ± 0.1† |
| CXCR-4 | 1.80 ± 0.12 | 2.42 ± 0.12†* | 1.12 ± 0.13 | 1.0 ± 0.11† |
The cells were purified from the spleen of mice (n = 8) and incubated without or with DHA (100 µM) for 4 h prior to the total RNA extraction. The mRNA expression of the genes was quantitatively determined by real-time PCR as described in Materials and Methods. *P < 0.01, significant differences between DHA-treated cells compared with untreated corresponding cells. †P < 0.01, significant differences between Teff and Treg cells.
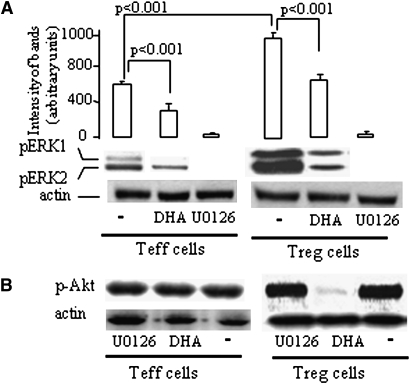
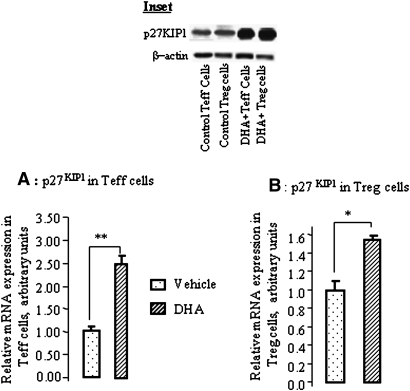
DHA modulates the expression of ERK1/2, Akt, and p27KIP1 in Treg and Teff cells
First, Western blotting experiments show that activated Treg cells expressed higher pERK1/2 than Teff cells. DHA significantly inhibited pERK1/2 in Teff and Treg cells (Fig. 6A). Interestingly, DHA inhibited the Akt phosphorylation only in Treg but not in Teff cells (Fig. 6B). Furthermore, the mRNA expression of p27KIP1 was upregulated by DHA both in Treg and Teff cells (Fig. 7A, B). DHA treatment also upregulated p27KIP1, in both types of cells, at the protein level (Fig. 7, inset).
Fig. 6.
DHA modulates ERK1/2 and Akt phosphorylation. Western blot analyses of the lysates of Teff and Treg cells, purified from the spleen of mice. The cells were activated with anti-CD3 and anti-CD28 antibodies for 20 min after their incubation with DHA (100 µM) or U0126 (5 µM). Blot shows one representative experiment reproduced at least four times. pERK1/2, phosphorylated ERK1/2; p-Akt, phosphorylated-Akt
Fig. 7.
DHA modulates p27KIP1 mRNA expression in Treg and Teff cells. The cells were purified from the spleen of mice and incubated without or with DHA (100 μM) for 4 h prior to the total RNA extraction from Teff (A) and Treg (B) cells. The p27KIP1 mRNA expression was quantitatively determined by real-time PCR as described in Materials and Methods. *P < 0.05 and **P < 0.01: significant differences between DHA-treated and untreated cells. Inset shows the p27KIP1 protein in Western blots before and after DHA treatment of Treg and Teff cells. The blots used for p27KIP1 were stripped off and reprobed for β -actin.
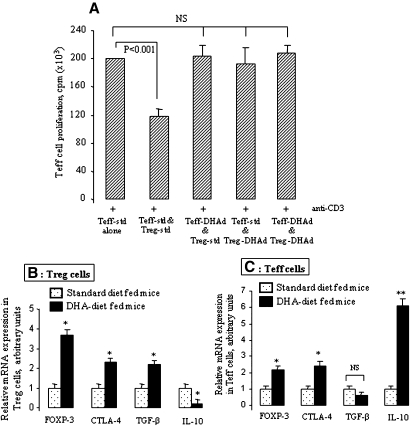
DHA-enriched diet curtails the suppressive capacity of Treg cells
To test whether DHA could influence, in vivo, the suppressive capacity of Treg cells on Teff cell proliferation, we first cocultured the Teff cells isolated from mice fed on a standard diet with the Treg cells isolated from mice fed on a DHA diet. Inversely, we cocultured Teff cells isolated from DHA-diet fed mice with the Treg cells isolated from standard diet fed mice. In both conditions, Treg cells again failed to suppress Teff cell proliferation (Fig. 8A). Interestingly, the Teff cell proliferation was not diminished when Treg and Teff cells, both from DHA-diet fed mice, were cocultured.
Fig. 8.
Effects of in vivo DHA supplementation on Treg and Teff cells. DHA-enriched diet abolished the suppressive capacity of Treg cells on Teff cell proliferation. A: The Teff and Treg cells were purified from the spleen of mice fed on a standard diet (as control group) or a DHA diet for 6 weeks; n = 10 mice per group of diet. The cells were cocultured with soluble anti-CD3 antibody for 46 h. In all experiments, cell proliferation was measured by [3H]thymidine incorporation. Results are expressed as mean ± SEM of experiment reproduced at least three times and performed in six replicates from different mice. NS, insignificant differences. B: DHA-enriched diet increases Foxp3, CTLA-4, and TGF-β mRNA expression in Treg cells. C: DHA-enriched diet enhances Foxp3, CTLA-4, and IL-10 mRNA expression in Teff cells. The Teff and Treg cells were purified from the spleen of mice fed on a standard diet (as control group) or a DHA diet for 6 weeks prior to the total RNA extraction; n = 10 mice per group of diet. The mRNA expression of the genes was quantitatively determined by real-time PCR as described in Materials and Methods. *P < 0.01 and **P < 0.001: significant differences between DHA diet-fed mouse cells compared with standard diet-fed mouse cells. Teff-std, Teff cells purified from mice fed on a standard diet; Teff-DHAd, Teff cells purified from mice fed on a DHA-diet; Treg-std, Treg cells purified from mice fed on a standard diet; Treg-DHAd, Treg cells purified from mice fed on a DHA diet.
DHA-enriched diet modulates the mRNA expression of Foxp3, CTLA-4, TGF-β, and IL-10 in T-cells
Similar to in vitro assays, we observed that, in the Treg cells isolated from mice fed on a DHA diet, the mRNA expression of Foxp3, CTLA-4, and TGF-β was upregulated, whereas that of IL-10 was downregulated (Fig. 8B). In Teff cells, DHA in vivo also induced the upregulation of the mRNA expression of Foxp3, CTLA-4, and IL-10, without any significant changes in TGF-β mRNA (Fig. 8C).
n-3 PUFA replace n-6 PUFA in the plasma membrane phospholipids of Treg and Teff cells
The DHA diet, rich in EPA and DHA, compared with the standard diet, leads to an increased incorporation of EPA and DHA (n-3 PUFA) into the plasma membrane phospholipids of Treg and Teff cells. This incorporation of n-3 PUFA was concomitant with a decrease in the proportions of AA (n-6 PUFA) in plasma membrane phospholipids of Treg and Teff cells of the mice fed on the DHA diet, compared with the cells of those fed on the standard diet (Table 4). The distribution of fatty acids in plasma membrane phospholipids of Treg and T eff cells was similar.
TABLE 4.
Percentage fatty acid composition of plasma membrane phospholipids of Treg cells purified from the mice spleen
| Fatty Acids (% of Total) | Cells from Mice Fed Standard Diet | Cells from Mice Fed DHA Diet |
|---|---|---|
| C16:0 | 1.99 ± 0.31 | 2.16 ± 0.39 |
| C16:1 | 27.01 ± 0.37 | 27.37 ± 0.20 |
| C18:0 | 18.79 ± 0.25 | 18.31 ± 0.67 |
| C18:1 | 13.15 ± 0.56 | 12.78 ± 0.75 |
| C18:2n-6 | 11.12 ± 0.75 | 10.19 ± 0.11 |
| C20:4n-6 (AA) | 25.67 ± 0.98 | 13.12 ± 0.28* |
| C20:5n-3 (EPA) | 0.41 ± 0.05 | 6.09 ± 0.15* |
| C22:6n-3 (DHA) | 1.77 ± 0.06 | 9.99 ± 0.41* |
The cells were purified from the spleen of mice fed a standard diet or DHA diet for 6 weeks. Values are mean ± SEM, n = 10 mice per group of diet. *P < 0.01, significant differences between DHA diet group and standard diet group. The distribution of the percentage of the fatty acid composition was statistically identical in Treg and Teff cells.
DISCUSSION
The identification of agents that control Treg cell differentiation and function is important to understand the host immune response in malignancy and autoimmunity. Treg cell suppressive activity has been partially attributed to IL-10 and CTLA-4, whereas their migration and adhesion have been attributed to the specific repertoire of chemokine receptors that they express (25).
Exogenous DHA or a DHA-rich diet inhibited the suppressive capacity of Treg cells on Teff cell proliferation. The exposure of Treg cells to DHA or in vivo enrichment with DHA upregulated TGFβ, but downregulated IL-10, mRNA expression in these cells, suggesting that DHA might be orienting Treg cell differentiation toward TH3 phenotype. Furthermore, the treatment of Teff cells with DHA or in vivo enrichment with DHA curtailed the Treg-induced inhibition of their proliferation. When the experiments were conducted on Treg and Teff cells both from DHA-diet fed mice, the Teff cell proliferation was not diminished by Treg cells.
DHA treatment of Treg or Teff cells upregulated Foxp3 both at mRNA and protein levels. In vivo enrichment with DHA of these cells also induced upregulation of the expression of Foxp3 and CTLA-4 mRNA. This observation is surprising as this fatty acid exerts, at one hand, an inhibitory action on Treg cell function and, on the hand, induces the expression of Treg cell markers. Hence, DHA influences two aspects of T-cells: expression of Treg cell markers and Treg cell activity. Before explaining the actions of DHA on these two aspects, we would like to cite a few exceptional examples that demonstrate that the upregulation of Treg markers is not necessarily linked to the upregulated Treg activity. Tran et al. (26) have shown that TGF-β treatment of human Teff (CD4+) cells induces high levels of Foxp3 and CTLA4 expression. Though Foxp3 expression was stable, the TGF-β-treated cells were neither anergic nor suppressive for Teff cell proliferation, suggesting that high levels of Foxp3 expression are insufficient to define a human CD4+Foxp3+ cell as a Treg cell, and other factors may be required to act in concert with Foxp3. Morgan et al. (27) have also shown that Foxp3 mRNA expression could be induced, in vitro, in human CD25−CD8+ cells; however, these cells failed to display Treg properties and exhibited a similar proliferative- and interferon-γ-secreting potential after antigenic stimulation. Wang et al. (28) have activated human CD4+ cells by plate-bound anti-CD3 and soluble anti-CD28 antibodies, in the presence of IL-2, and they noticed that Foxp3 is expressed in a high percentage of activated T-cells; however, these CD4+CD25+Foxp3+ cells, being hyporesponsive, failed to display suppressive function like natural Treg cells.
One of the possible mechanisms of upregulating the Foxp3 by DHA might be its action on the downregulation of Smad7 activity. In fact, it has been shown that TGF-β upregulates Foxp3 expression by inhibiting Smad7 activity (24). Similarly, in our study, we noticed that DHA downregulated the Smad7 activity in Treg and Teff cells. Since DHA-treated Treg cells express high levels of TGF-β mRNA, it is possible that TGF-β might be secreted in high quantities by these cells, and once TGF-β is present in the culture medium, this cytokine might exert an autocrine action and induce high expression of Foxp3 by downregulating the Smad7 levels. It is also possible that DHA is directly acting on the Smad7. Moreover, DHA-induced expression of CTLA-4 corroborates the study of Ly et al. (29), who reported that a DHA-enriched diet increased the expression CTLA-4 on murine T-cells.
The Teff cells highly expressed both Foxp3 and IL-10 mRNA after DHA treatment. Hence, it is possible that IL-10, secreted into the extracellular environment, might exert an action in an autocrine fashion. Indeed, IL-10 has been shown to promote the expression of the Foxp3 (30). Glucocorticoids that are the inducers of IL-10 have been shown to induce the expression of Foxp3 during a local and systemic treatment with these agents in asthmatic subjects (31). However, nothing is known regarding the Smad7- and IL-10-induced expression of Foxp3. IL-10 has been shown to modulate Smad7 levels in human bronchial epithelial cells (32). However, the so-called Treg-like Teff cells did not acquire true Treg cell suppressive functions, though these Teff cells, treated with DHA, expressed Treg markers. Besides, these Treg-like Teff cells (treated with DHA) are not anergic as is the case of Treg cells that could not be proliferated by exogenous IL-2. In fact, exogenous IL-2 could induce the proliferation of the Teff cells treated by DHA. We have previously demonstrated DHA-treated T-cell proliferation by inhibiting the transcription of IL-2 gene in Jurkat T-cells (33). Besides, McMurray et al. (34) have shown that reduction in T-cell proliferative capacity, by DHA, was accompanied by reductions in IL-2 secretion, suggesting that dietary n-3 fatty acids act, in part, by interrupting the autocrine IL-2 activation pathway. In DHA-treated Teff cells, a stimulatory effect of IL-2 may be evoked via its action on IL-2R.
As far as the decrease in Treg cell functions by the DHA is concerned, we can hypothesize that this fatty acid may be downregulating the Treg cell activity by interfering with the critical downstream components of the Foxp3-driven suppressor pathway. In fact, it is now becoming clear that interaction of Foxp3 with other transcription factors (NAFT or Runx-1) or histone decaetyltransferase and class II histone deacetylase is critical for the repression of the transcription of IL-2 gene by Foxp3 (35). Li et al. (35) have shown that transcriptional repression by Foxp3 involves a histone acetyltransferase-deacetylase complex that includes TIP60 (Tat-interactive protein, 60 kDa) and class II histone deacetylases, HDAC7. Our findings corroborate these results as we noticed that Treg cells express ensemble acetylated lysine residues and HDAC7 in Foxp3-immunopreciptated nuclear proteins. However, DHA-induced upregulation of HDAC7 and dwonregulation of acetylated lysine residues in Teff cells might be responsible for the inhibition of Teff cell growth (caused by high HDAC7 levels), thus conferring them hyporesponsive. The Treg cells while treated with DHA lose their ensemble complex, as this fatty acid diminishes the acetylation in these cells. However, it remains to be ascertained how Smad7 and Foxp3-HDAC7 are differently regulated, and this aspect might be the subject of future studies.
Because the upregulated expression of HDAC7 by DHA is found associated with downregulated activity of Treg cells, we employed TSA, an inhibitor of HDAC activity. We noticed that TSA upregulated the Treg suppressive capacity, and TSA-treated Treg cells remained anergic (data not shown). These observations are in accordance with a number of studies that have shown that TSA upregulates Treg cell functions in NZB/W F1 (36) and Balb/c mice (37). Nonetheless, in both the studies, TSA failed to influence the IL-2 gene expression and, therefore, to reverse the Treg cell anergy (36, 37). As far as DHA is concerned, we observed that DHA diminished the upregulated TSA-induced Treg activity, though this fatty acid could not completely reverse the Treg activity. This partial effect of DHA might be due to the fact that the cells were first incubated with TSA and then with DHA.
The suppressive capacity of Treg cells depends on their ability to migrate and enter the tissues, and this might help prevent autoimmune disease progression (9). This trafficking ability of Treg cells depends on their capacity to express migratory and adhesion factors. By analyzing the expression of the chemokine receptors (needed for their migratory ability), we observed that Treg cells expressed higher CCR-4, CCR-8, and CXCR-4 mRNA than Teff cells as it has been reported in previous studies in mice (9) and human (25). As far as DHA is concerned, we observed that the exposure of Treg cells to DHA significantly reduced their ability to migrate toward MDC and SDF-1. In an attempt to link the expression of the chemokine receptors, we observed that DHA curtailed the expression of CCR-4 and CXCR-4 mRNA in Treg cells. It is noteworthy that DHA did not affect the migration of Teff cells, and this observation is in accordance with a study that has shown that some agents, like dopamine, only affected the migration of Treg cells without influencing the same in Teff cells (9). Since Treg cells highly express L-selectin, which is essential for their migration and normal tissue distribution (38), it is possible that DHA is inhibiting their migration via inhibition of expression of L-selectin. Indeed, we noticed that DHA curtailed significantly the expression of L-selectin mRNA in Treg cells. These observations again explain the diminished migration of Treg cells even in the absence of MDC and SDF-1. Our observations corroborate the report of Collie-Duguid and Wahle (39) who have shown that both DHA and EPA transcriptionally decrease the expression of adhesion molecules, such as P-selectin and ICAM-1, induced by cytokines in several cell lines. As anticipated, the Teff cells did not express L-selectin mRNA in high quantities and DHA did not affect the same.
T-cell migration and adhesion have been linked to the phosphorylation of ERK1/2 activation (40). Moreover, Treg cell activity has been found to be associated with ERK1/2 activation (9). It is interesting to mention that activated Treg cells expressed more phosphorylated ERK1/2 than activated Teff cells. This observation is in close agreement with the findings of Kipnis et al. (9) who have shown that Treg activity is associated with ERK1/2 phopshorylation and the degree of phosphorylation is always higher in Treg cells than that in Teff cells. Furthermore, DHA was found to significantly diminish ERK1/2 phosphorylation in activated Treg and Teff cells. These observations corroborate our previous reports in which we have shown that DHA inhibits significantly ERK1/2 phosphorylation in Jurkat T-cells (11, 41). Keeping in view the present results, we can conclude that DHA downregulated Treg cell activity, in part, by reducing ERK1/2 activation. Besides, U0126, an inhibitor of ERK1/2 phosphorylation, also inhibited the suppressive activity of Treg cells (data not shown).
Besides ERK1/2, the phosphatidylinositol-3-kinase (PI3K) and Akt/protein kinase B (hence referred to as Akt) play a critical role in T-cell survival, expansion, and differentiation (42). Phosphatidylinositol-3-kinase-Akt is indispensable for cell cycle progression. To see whether this pathway is also altered in Treg cells, we determined Akt phosphorylation in Treg and Teff cells. U0126, an inhibitor of MEK, did not influence Akt phosphorylation; however, DHA diminished the same in Treg, but not in Teff, cells, suggesting that this fatty acid also controls cell cycle progression via its action on Akt phosphorylation.
Because ERK1/2 and Akt phosphorylation control the expression of p27KIP1, an inhibitor of cyclinE/cyclinD kinase 2 that regulates the G1 phase of the cell cycle (43), we were tempted to assess the expression of 27KIP1. We observed that DHA increased the p27KIP1 expression, both at mRNA and protein levels, in Treg and Teff cells. Our observations are in contradiction with the results of De Rosa et al. (44), who have shown the downregulation of p27KIP1 and increased expression of Foxp3 in Treg cells. However, these authors associated the downregulation of p27KIP1 with the reversal of the anergic state of Treg cells. Besides, in that study, downregulation of p27KIP1 was associated with increased phosphorylation of ERK1 and ERK2. On the other hand, it has been shown that induction and maintenance of the state of anergy of Treg cells is controlled by an increased expression of p27KIP1 (45), and this finding supports our observations on the DHA-induced increased expression of p27KIP1, associated with the anergic state of Treg cells and reduced phosphorylation of ERK1 and ERK2. These observations partly corroborate our recent findings where we have shown that DHA, by inhibiting ERK1/2 phosphorylation, upregulates the p27KIP1 protein and blocks mouse mammary cancer cells in the late-G1 phase of the cell cycle (11). Moreover, it is possible that Foxp3 expression and downregulation of p27KIP1 might be two different mechanisms where the latter controls anergic state of the cells.
As far as the in vivo effects of DHA are concerned, the analysis of fatty acid composition of the plasma membranes showed that feeding of a DHA diet leads to an increased incorporation of EPA and DHA into the plasma membrane phospholipids of Treg and Teff cells. This incorporation of n-3 PUFA results in a decrease of AA (n-6 PUFA). Hence, we can state that DHA influences the Treg cell activity by being incorporated into their plasma membranes. Our observations corroborate our previous findings (43, 46), wherein we have shown that dietary n-3 PUFA were significantly incorporated into the plasma membranes and, consequently, modulate T-cell activation. However, the mechanism of action of in vivo PUFA enrichment of T-cell phospholipids can be explained on the basis of the reports that have shown that incubation of T-cells with PUFA leads to the displacement of Src family protein tyrosine kinase from lipid rafts in parallel with the inhibition of calcium response (47, 48). This could be the one of the mechanisms underlying PUFA-mediated modulation of T-cell signaling, which may explain the decreased activation of Treg cells. Besides, we have previously shown that plasma membrane DHA enrichment modulates the downstream cell signaling that ultimately influences the expression of IL-2 gene (12, 19). Therefore, it is tempting to assume that DHA via its incorporation might be influencing the genes involved in the regulation of T-cell functions.
Although this study raises more questions than answers on the role of DHA in the modulation of Treg cell function, to our knowledge, ours is the first study that demonstrates that DHA decreased Treg cell suppressive function by reducing their migratory abilities via ERK1/2 and Akt pathways and acetylation/decaetylation of nuclear histones.
Acknowledgments
The authors thank the French Ministry of Higher Education and Research, which sanctioned the contingent grants for this work.
Footnotes
Abbreviations:
- AA
- arachidonic acid
- CFSE
- carboxyfluorescien succinimidyl ester
- CTLA-4
- cytotoxic T lymphocyte-associated antigen-4
- DHA
- docosahexaenoic acid
- EPA
- eicosapentaenoic acid
- ERK
- extracellular signal-regulated kinase
- Foxp3
- forkhead/winged helix transcription factor protein-3
- HDAC
- histone desacetylase
- IL
- interleukin
- iTreg
- inducible CD4+CD25+ regulatory T-cells
- MDC
- macrophage-derived chemokines
- OD
- optical density
- PGE2
- prostaglandin E2
- PI3K
- phosphatidylinositol-3-kinase
- SDF-1
- stroma cell-derived factor-1
- Teff
- CD4+CD25− effector T-cells
- TGF-β
- transforming growth factor
- Treg
- CD4+CD25+ regulatory T-cells
- TSA
- trichostatin A
This work was supported by a contingent grant from the Ministry of Higher Education and Research, France.
REFERENCES
- 1.Sakaguchi S.2000. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 101: 455–458 [DOI] [PubMed] [Google Scholar]
- 2.Shevach E. M.2002. CD4+ CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2: 389–400 [DOI] [PubMed] [Google Scholar]
- 3.Brunkow M. E., Jeffery E. W., Hjerrild K. A., Paeper B., Clark L. B., Yasayko S. A., Wilkinson J. E., Galas D., Ziegler S. F., Ramsdell F. 2001. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 27: 68–73 [DOI] [PubMed] [Google Scholar]
- 4.Hori S., Sakaguchi S. 2004. Foxp3: a critical regulator of the development and function of regulatory T cells. Microbes Infect. 6: 745–751 [DOI] [PubMed] [Google Scholar]
- 5.Bluestone J. A., Abbas A. K. 2003. Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 3: 253–257 (Review) [DOI] [PubMed] [Google Scholar]
- 6.Groux H.2001. An overview of regulatory T cells. Microbes Infect. 3: 883–889 [DOI] [PubMed] [Google Scholar]
- 7.Buckner J. H., Ziegler S. F. 2004. Regulating the immune system: the induction of regulatory T cells in the periphery. Arthritis Res. Ther. 6: 215–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baratelli F., Lin Y., Zhu L., Yang S. C., Heuzé-Vourc'h N., Zeng G., Reckamp K., Dohadwala M., Sharma S., Dubinett S. M. 2005. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J. Immunol. 175: 1483–1490 [DOI] [PubMed] [Google Scholar]
- 9.Kipnis J., Cardon M., Avidan H., Lewitus G. M., Mordechay S., Rolls A., Shani Y., Schwartz M. 2004. Dopamine, through the extracellular signal-regulated kinase pathway, downregulates CD4+CD25+ regulatory T-cell activity: implications for neurodegeneration. J. Neurosci. 24: 6133–6143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heller A. R., Rossel T., Gottschlich B., Tiebel O., Menschikowski M., Litz R. J., Zimmermann T., Koch T. 2004. Omega-3 fatty acids improve liver and pancreas function in postoperative cancer patients. Int. J. Cancer. 111: 611–616 [DOI] [PubMed] [Google Scholar]
- 11.Khan N. A., Nishimura K., Aires V., Yamashita T., Oaxaca-Castillo D., Kashiwagi K., Igarashi K. 2006. Docosahexaenoic acid inhibits cancer cell growth via p27Kip1, CDK2, ERK1/ERK2, and retinoblastoma phosphorylation. J. Lipid Res. 47: 2306–2313 [DOI] [PubMed] [Google Scholar]
- 12.Denys A., Hichami A., Khan N. A. 2005. n-3 PUFAs modulate T-cell activation via protein kinase C-alpha and -epsilon and the NF-kappaB signaling pathway. J. Lipid Res. 46: 752–758 [DOI] [PubMed] [Google Scholar]
- 13.Arrington J. L., Chapkin R. S., Switzer K. C., Morris J. S., McMurray D. N. 2001. Dietary n-3 polyunsaturated fatty acids modulate purified murine T-cell subset activation. Clin. Exp. Immunol. 125: 499–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanderson P., Calder P. C. 1998. Dietary fish oil diminishes lymphocyte adhesion to macrophage and endothelial cell monolayers. Immunology. 94: 79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu J., Xu D., Liu Z., Shi M., Zhao P., Fu B., Zhang Z., Yang H., Zhang H., Zhou C., et al. 2007. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 132: 2328–2339 [DOI] [PubMed] [Google Scholar]
- 16.Kono K., Kawaida H., Takahashi A., Sugai H., Mimura K., Miyagawa N., Omata H., Fujii H. 2006. CD4+CD25 high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol. Immunother. 55: 1064–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curiel T. J., Coukos G., Zou L., Alvarez X., Cheng P., Mottram P., Evdemon-Hogan M., Conejo-Garcia J. R., Zhang L., Burow M., et al. 2004. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 10: 942–949 [DOI] [PubMed] [Google Scholar]
- 18.Dignam J. D., Lebovitz R. M., Roeder R. G. 1983. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11: 1475–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Triboulot C., Hichami A., Denys A., Khan N. A. 2001. ω-3 dietary polyunsaturated fatty acids diminish hypertension: implication of T-cell signaling mechanisms. J. Nutr. 131: 2364–2369 [DOI] [PubMed] [Google Scholar]
- 20.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917 [DOI] [PubMed] [Google Scholar]
- 21.Slover H. T., Lanza E. 1979. Quantitative analysis of food fatty acids by capillary gas chromatography. J. Am. Oil Chem. Soc. 56: 933–943 [Google Scholar]
- 22.Ramsdell F. 2003. Foxp3 and natural regulatory T cells: key to a cell lineage? Immunity. 19: 165–168 [DOI] [PubMed] [Google Scholar]
- 23.Dieckmann D., Plottner H., Berchtold S., Berger T., Schuler G. 2001. Ex vivo isolation and characterization of CD4+CD25+ T cells with regulatory properties from human blood. J. Exp. Med. 193: 1303–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fantini M. C., Becker C., Monteleone G., Pallone F., Galle P. R., Neurath M. F. 2004. TGF-b induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 172: 5149–5153 [DOI] [PubMed] [Google Scholar]
- 25.Sebastiani S., Allavena P., Albanesi C., Nasorri F., Bianchi G., Traidl C., Sozzani S., Girolomoni G., Cavani A. 2001. Chemokine receptor expression and function in CD4+ T lymphocytes with regulatory activity. J. Immunol. 166: 996–1002 [DOI] [PubMed] [Google Scholar]
- 26.Tran D. Q., Ramsey H., Shevach E. M. 2007. Induction of FOXP3 expression in naive human CD4+FOXP3– T cells by T-cell receptor stimulation is transforming growth factor-β–dependent but does not confer a regulatory phenotype. Blood. 110: 2983–2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan M. E., van Bilsen J. H. M., Bakker A. M., Heemskerk B., Schilham M. W., Hartgers F. C., Elferink B. G., van den Zanden L., de Vries R. R. P., Huizinga T. W. J., et al. 2005. Expression of FOXP3 mRNA is not confined to CD4+ CD25+ T regulatory cells in humans. Hum. Immunol. 66: 13–20 [DOI] [PubMed] [Google Scholar]
- 28.Wang J., Ioan-Facsinay A., van der Voort E. I., Huizinga T. W., Toes R. E. 2007. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur. J. Immunol. 37: 129–138 [DOI] [PubMed] [Google Scholar]
- 29.Ly L. H., Smith R., Switzer K. C., Chapkin R. S., McMurray D. N. 2006. Dietary eicosapentaenoic acid modulates CTLA-4 expression in murine CD4+ T-cells. Prostaglandins Leukot. Essent. Fatty Acids. 74: 29–37 [DOI] [PubMed] [Google Scholar]
- 30.Fontenot J. D., Gavin M. A., Rudensky A. Y. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4: 330–336 [DOI] [PubMed] [Google Scholar]
- 31.Karagiannidis C., Akdis M., Holopainen P., Woolley N. J., Hense G., Ruckert B., Mantel P. Y., Menz G., Akdis C. A., Blaser K., et al. 2004. Glucocorticoids regulate FOXP3 expression and regulatory T cells in asthma. J. Allergy Clin. Immunol. 114: 1425–1433 [DOI] [PubMed] [Google Scholar]
- 32.Fueki N., Sagara H., Akimoto K., Ota M., Okada T., Sugiyama K., Fueki M., Makino S., Fukuda T. 2007. Interleukin-10 regulates transforming growth factor-beta signaling in cultured human bronchial epithelial cells. Respiration. 74: 454–459 [DOI] [PubMed] [Google Scholar]
- 33.Denys A., Hichami A., Khan N.A. 2002. Eicosapentaenoic acid and docosahexaenoic acid modulate MAP kinase enzyme activity in human T-cells. Mol. Cell Biochem. 232:143–148 [DOI] [PubMed] [Google Scholar]
- 34.McMurray D. N., Jolly C. A., Chapkin R. S. 2000. Effects of dietary n-3 fatty acids on T cell activation and T cell receptor mediated signaling in a murine model. J. Infect. Dis. 182: S103–S107 [DOI] [PubMed] [Google Scholar]
- 35.Li B., Samanta A., Song X., Iacono K. T., Bembas K., Tao R., Basu S., Riley J. L., Hancock W. W., Shen Y., et al. 2007. FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. Proc. Natl. Acad. Sci. USA. 104: 4571–4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reilly C. M., Thomas M., Jr. Gogal R., Olgun S., Santo A., Sodhi R., Samy E. T., Peng S. L., Gilkeson G. S., Mishra N. 2008. The histone deacetylase inhibitor trichostatin A upregulates regulatory T cells and modulates autoimmunity in NZB/W F1 mice. J. Autoimmun. 31: 123–130 [DOI] [PubMed] [Google Scholar]
- 37.Tao R., de Zoeten E. F., Ozkaynak E., Chen C., Wang L., Porrett P. M., Li B., Turka L. A., Olson E. N., Greene M. I., et al. 2007. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat. Med. 13: 1299–1307 [DOI] [PubMed] [Google Scholar]
- 38.Venturi G. M., Conway R. M., Steeber D. A., Tedder T. F. 2007. CD25+CD4+ regulatory T cell migration requires L-selectin expression: L-selectin transcriptional regulation balances constitutive receptor turnover. J. Immunol. 178: 291–300 [DOI] [PubMed] [Google Scholar]
- 39.Collie-Duguid E. S., Wahle K. W. 1996. Inhibitory effect of fish oil N-3 polyunsaturated fatty acids on the expression of endothelial cell adhesion molecules. Biochem. Biophys. Res. Commun. 220: 969–974 [DOI] [PubMed] [Google Scholar]
- 40.Wing K., Suri-Payer E., Rudin A. 2005. CD4+CD25+-regulatory T cells from mouse to man. Scand. J. Immunol. 62: 1–15 [DOI] [PubMed] [Google Scholar]
- 41.Denys A., Aires V., Hichami A., Khan N. A. 2004. Thapsigargin-stimulated MAP kinase phosphorylation via CRAC channels and PLD activation: inhibitory action of docosahexaenoic acid. FEBS Lett. 564: 177–182 [DOI] [PubMed] [Google Scholar]
- 42.Li L., Godfrey W. R., Porter S. B., Ge Y., June C. H., Blazar B. R., Boussiotis V. A. 2005. CD4+CD25+ regulatory T-cell lines from human cord blood have functional and molecular properties of T-cell anergy. Blood. 106: 3068–3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sherr C. J. 1996. Cancer cell cycles. Science. 274: 1672–1677 [DOI] [PubMed] [Google Scholar]
- 44.De Rosa V., Procaccini C. C., Calì G., Pirozzi G., Fontana S., Zappacosta S., La Cava A., Matarese G. 2007. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 26: 241–255 [DOI] [PubMed] [Google Scholar]
- 45.Adler H. S., Kubsch S., Graulich E., Ludwig S., Knop J., Steinbrink K. 2007. Activation of MAP kinase p38 is critical for the cell-cycle-controlled suppressor function of regulatory T cells. Blood. 109: 4351–4359 [DOI] [PubMed] [Google Scholar]
- 46.Guermouche B., Yessoufou A., Soulaimann N., Merzouk H., Moutairou K., Hichami A., Khan N. A. 2004. (N-3) fatty acids modulate T-cell calcium signaling in obese macrosomic rats. Obes. Res. 12: 1744–1753 [DOI] [PubMed] [Google Scholar]
- 47.Zeyda M., Staffler G., Horejsi V., Waldhausl W., Stulnig T. M. 2002. LAT displacement from lipid rafts as a molecular mechanism for the inhibition of T cell signaling by polyunsaturated fatty acids. J. Biol. Chem. 277: 28418–28423 [DOI] [PubMed] [Google Scholar]
- 48.Stulnig T. M., Berger M., Sigmund T., Raederstorff D., Stockinger H., Walhausl W. 1998. Polyunsaturated fatty acids inhibit T cell signal transduction by modification of detergent-insoluble membrane domains. J. Cell Biol. 143: 637–644 [DOI] [PMC free article] [PubMed] [Google Scholar]