Abstract
Our recent studies showed that transglutaminase-1 (TGase-1) is uniquely expressed in mouse renal proximal tubular cells (RPTC) and mediates cell proliferation. In this study, we investigated the role of TGase-1 in cell survival and the survival signaling pathways regulated by TGase-1 in RPTC following oxidant injury. Exposure of RPTC to hydrogen peroxide (H2O2) resulted in apoptosis and an increase in TGase activity. Inhibition of TGase activity with monodansylcadervine (MDC), a TGase inhibitor, or knockdown of TGase-1 with small interference (si)RNA enhanced apoptosis and decreased cell survival in H2O2-treated RPTC. Conversely, overexpression of TGase-1 rendered RPTC more resistant to H2O2 toxicity and MDC treatment blocked this response. Concurrent with RPTC apoptosis, phosphorylation of AKT, signal transducer and activator of transcription-3 (STAT3), and glucogen synthase kinase-3β (GSK-3β) were observed. Pretreatment of cells with MDC or TGase-1 siRNA inhibited phosphorylation of all these molecules. Inhibition of either the AKT or STAT3 pathway potentiated H2O2-induced cell death and increased GSK-3β activity by dephosphorylation at serine 9. Furthermore, treatment with GSK-3β inhibitors reduced H2O2-induced apoptosis and abolished the death-promoting effect of AKT and STAT3 inhibition. Therefore, we have identified TGase-1 as a novel survival factor in renal epithelial cells and it contributes to cell survival through activation of the AKT and STAT3 signaling pathways following oxidant injury.
Keywords: renal proximal tubular cells
acute kidney injury (AKI) is a complication that occurs frequently in hospitalized patients. Most cases of AKI arise from ischemia/reperfusion (I/R) injury. The pathophysiology of I/R-induced AKI involves a complex interplay between hemodynamics, tubular injury, and inflammation (20, 31, 39). At the tubular level, injury occurs predominantly to the proximal tubule, leading to cell death. The excessive formation of reactive oxygen species (ROS) including hydrogen peroxide (H2O2) during I/R injury contributes to the pathogenesis of AKI (5, 31, 36).
Multiple signal transduction pathways that serve to coordinate the cellular response and ultimately determine cell fate are activated by oxidant injury. It is well documented that H2O2 can induce activation of phosphoinositide 3-kinase (PI3K)/AKT and the Janus-activated kinase 2/signal transducer and activator of transcription-3 (JAK2-STAT3) pathways, which are commonly involved in the development of oxidant resistance (26, 31, 37, 41). Activation of the PI3K/AKT pathway subsequently leads to phosphorylation of a number of apoptosis-regulatory molecules including glycogen synthase kinase-3β (GSK-3β; Ref. 10). Phosphorylation of GSK-3β by AKT at serine 9 reduces its kinase activity and offers cell survival advantage (24).
Increasing evidence suggests that transglutaminases, a family of Ca2+-dependent enzymes, are also involved in cell survivals. For example, Antonyak et al. (4) showed that transglutaminase-2 (TGase-2) protects NIH3T3 fibroblasts from apoptosis induced by serum deprivation. Further, TGase-2 activation promotes cell survival against various apoptotic stimuli and toxicants like doxorubicin and cisplatin in cancer cell lines (3, 4, 11, 12). Datta et al. (13) showed that TGase-2 affords cell survival advantage against doxorubicin-induced apoptosis in SKBR3 cells. The mechanism underlying TGase-mediated survival remains poorly understood. The best characterized function of TGases is as a transamidating acyltransferase that cross-links glutamine with lysine residues (19, 44). TGase-2-mediated cross-linking of caspase-3 resulted in inhibition of caspase-3 activity and suppression of apoptosis in thapsigargin-treated Bax-deficient HCT116 cells (46). TGase-2 has also been shown to have intrinsic kinase activity, functioning as a serine/threonine kinase to induce phosphorylation of some proteins such as insulin-like growth factor-binding protein-3 (IGFBP-3) and retinoblastoma protein (Rb; Refs. 28–30). In addition, TGase-2 mediates the activation of PI3K/AKT pathway in tumor cells (6).
Although nine members of the TGases family (TGase-1-7, coagulation factor XIIIα, and band 4.2; Ref. 18) have been identified, the functional significance of TGases in AKI has not been studied yet. Recently, we demonstrated that TGase-1, but not -2, -5 and -7, are expressed in renal epithelial cells and is required for proliferation. TGase-1-mediated renal proximal tubular cells (RPTC) proliferation occurs through the JAK2-STAT3 signaling pathway (49). In this study, we examined the role of TGase-1 in the regulation of renal proximal tubule cell survival and the pathway by which it promotes the cell survival following oxidative stress.
MATERIALS AND METHODS
Chemicals and antibodies.
Antibodies to phospho-STAT3 (Tyr705), STAT3, phospho-AKT(Ser 473), AKT, phospho-GSK-3β (serine 9), and GSK-3β were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibody to TGase-1 was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The small interference RNA (siRNA) specific for TGase-1 or STAT3 and lipofectamine were purchased from Invitrogen (Carlsbad, CA, USA). Plasmid encoding the mouse TGase-1 gene pCMV6-TGase-1 was purchased from Origene (Rockville, MD). LY294002 was obtained from Biomol (Plymouth Meeting, PA), and thiadiazoldione-8 (TDZD-8) and S3I201 were obtained from Calbiochem (San Diego, CA). The 5-(biotinamido)pentylamine was purchased from GE Healthcare (Piscataway, NJ). The in situ cell death detection kit [terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling technology (TUNEL) technology] was purchased from Roche Diagnostics (Mannheim, Germany). All other chemicals and reagents were purchased from Sigma.
Cells and treatments.
Immortalized mouse RPTC were kindly provided by Dr. Elsa Bella-Reuss and were cultured in DMEM/F-12 with 5% FBS at 37°C in 5% CO2. This cell line expresses P-glycoprotein and has a brush border, and a conserved epithelial morphology (14) has been well characterized. In all the experiments, RPTC were starved for 24 h with serum-free DMEM/F-12 before treatment with 1 mM H2O2 to induce apoptosis. When various pharmacological inhibitors were used, the same volume of DMSO was added to control samples.
Transfection of siRNA and plasmids into cells.
The siRNA oligonucleotides targeted specifically to mouse TGase-1 or STAT3 were used in this experiment. siRNA (750 pmol) was transfected into RPTC (2 × 106) using the Nucleofector Kit V and the Amaxa Nucleofector device according to the manufacturer's instructions (Gaithersburg, MD). In parallel, 750 pmol of scrambled siRNA were used to control for off-target changes in RPTC. After transfection, cells were cultured in DMEM/F-12 for 12 h and then switched to a serum-free medium for an additional 24 h.
For transfection of plasmids, plasmid DNA and lipofectamine (Invitrogen) were diluted separately in a serum-free medium and incubated at room temperature for 5 min. After incubation, the diluted DNA and lipofectamine were mixed and incubated at room temperature for 20 min. Aliquots of the transfection mixture were added to each well of the cell culture plate. Five hours after transfection, the medium was replaced with a fresh culture medium and cultured for 12 h. The cells were then starved with serum-free DMEM/F-12 for 24 h.
Apoptosis detection by TUNEL staining.
TUNEL was used for detection of apoptosis at single cell level, based on labeling of free 3'-OH terminal in DNA strand breaks. The cells were stained with TUNEL according to the manufacturer's directions and examined with a fluorescent microscope in a green light and photographed at ×200.
Determination of cell viability by MTT assay.
Cell viability was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. After treatment, MTT was added (final concentration, 0.5 mg/ml) and incubated for 1 h. Tetrazolium released by the addition of DMSO, and the optical density was determined with a spectrophotometer at 570-nm reader (Molecular Devices, Sunnyvale, CA).
Nuclear staining.
After treatment, cells were washed with PBS, fixed in methanol, and then stained with DAPI. Cells with condensed nuclei and/or DNA fragmentation were considered to be apoptotic. Cells in four random fields (×200) of each sample were counted, and three independent experiments were conducted in triplicate.
In situ transamidation assays.
TGase transamidation activity was evaluated by determining the incorporation of 5-(biotinamido)pentylamine (BP) using horseradish peroxidase- conjugated streptavidin according to the procedures described by Shin et al. (39a). Briefly, serum-starved RPTC were treated with H2O2 for different time points (10–120 min) and also treated with 100 μM of MDC for 1 h before treatment with H2O2 for 30 min. RPTC were labeled with 1 mM BP for 1 h before harvesting, and the cell extracts were prepared by brief sonication, followed by centrifugation at 13,000 rpm for 10 min at 4°C. Immunoblot analysis was performed by subjecting 30 μg of cell extracts to SDS-PAGE using a 10% gel, and the proteins were transferred to the polyvinylidene difluoride membrane. The proteins incorporated with BP were probed with horseradish peroxidase-conjugated streptavidin and visualized by chemiluminescence detection.
Immunoblot analysis.
After various treatments, cells were washed once with ice-cold PBS and harvested in a cell lysis buffer. Proteins (20 μg) were separated by SDS-PAGE and transferred to nitrocellulose membranes. After incubation with 5% skim milk overnight at 4°C, membranes were incubated with a primary antibody for 1 h at room temperature and then incubated with appropriate horseradish peroxidase-conjugated secondary antibody for an additional 1 h. Bound antibodies were visualized by chemiluminescence detection.
Statistical analysis.
Data are presented as means ± SD and were subjected to one-way ANOVA. Multiple means were compared using Tukey's test, and differences between two groups were determined by Student's t-test. P < 0.05 was considered statistically significant.
RESULTS
Activation of TGase-1 is required for RPTC survival following oxidant injury.
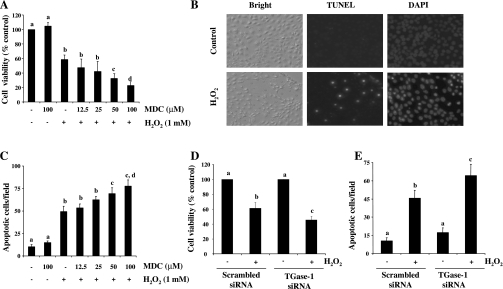
Intracellular ROS has been reported to be involved in the activation of TGases (6, 19). However, the role of TGases in RPTC death following oxidant injury is not clear. To address this issue, RPTC were exposed to 1 mM H2O2 in the presence or absence of MDC, a pseudosubstrate inhibitor of TGases that is widely used for inhibition of TGase activity (4, 49), and cell viability was examined using the MTT assay. Cell viability was decreased to 60% in RPTC treated with H2O2 alone for 4 h and further reduced to 38 and 25% in the presence of 50 and 100 μM MDC, respectively (Fig. 1A). H2O2-induced cell death was characterized by shrunken nuclei and positive TUNEL staining, the hallmarks of apoptosis, and the presence of MDC dose dependently enhanced H2O2-induced apoptosis (Fig. 1, B and C).
Fig. 1.
Effect of transglutaminase-1 (TGase-1) inhibition on H2O2-induced apoptosis in RPTC. Renal proximal tubular cells (RPTC) cells were treated with monodansylcadervine (MDC; 12.5–100 μM) for 1 h and then exposed to 1 mM of H2O2 for 4 h. Cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (A). DAPI and terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling technology (TUNEL) staining was performed and photographed (B). Cells stained with DAPI and apoptotic cells were counted from 4 different fields in each sample (×200; C). RPTC were transfected with scrambled small interference (si)RNA or TGase-1-specific small interference (si)RNA. At 12 h after transfection, cells were starved for an additional 24 h and then treated with 1 mM H2O2 for 4 h. Cell viability and apoptosis were assessed by MTT assay (D) and DAPI staining (E), respectively. Values are means ± SD of 3 independent experiments conducted in triplicates and expressed as the percentage of control (A and D) or total apoptotic cells per field (C and E). Bars with different letters are significantly different from one another (P < 0.05).
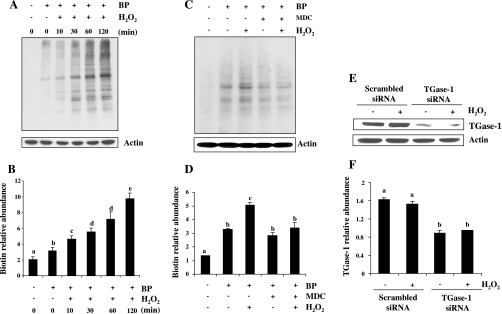
To determine the effect of MDC on H2O2-induced TGase activity, TGase activity was measured by labeling TGase-specific substrates with the biotin-labeled primary BP (35) followed by analyzing the cross-linked products by immunoblotting and horseradish peroxidase-conjugated streptavidin. As shown in Fig. 2, C and D, the basal level of TGase activity was detectable and H2O2 exposure resulted in a time-dependent increase in TGase activity, which was observed at 10 min and the maximal at 120 min (Fig. 2, A and B). In the presence of 100 μM MDC, H2O2-induced TGase activity was inhibited to the basal level (Fig. 2, C and D). These data suggest that the basal level of TGase activity is not sufficient for protecting cells from apoptosis and H2O2-stimulated TGase activity is required for cell survival in RPTC.
Fig. 2.
Inhibition of TGase activity by MDC and downregulation of TGase-1 by siRNA in cultured RPTC following H2O2 exposure. RPTC were incubated with 1 mM 5-(biotinamido)pentylamine (BP) for 1 h and then treated with 1 mM of H2O2 for indicated time (A) or 30 min (C) in the presence or absence of MDC (100 μM). Cells were harvested for analysis of TGase transamidation activity as described in materials and methods. A and C: representative blots for TGase activity from 3 independent experiments. B and D: biotin-labeled proteins in A and C were quantified by densitometry and normalized to actin. RPTC were transfected with scrambled siRNA or TGase-1-specific siRNA. At 12 h after transfection, cells were starved for an additional 24 h and then treated with 1 mM H2O2 for 30 min (E and F). Cell lysates were prepared and subjected to immunoblot analysis with antibodies for TGase-1 and actin (E). The level of TGase-1 was quantified by densitometry and normalized to actin level (F). Values are means ± SD of 3 independent experiments. Bars with different letters are significantly different from one another (P < 0.05).
Our recent study (49) shows that TGase-1, but not -2, -5, and -7, is expressed in RPTC, suggesting that H2O2-induced TGase activity is attributed to TGase-1. To elucidate the role of TGase-1 in cell survival following oxidant injury, we examined the effect of downregulation of TGase-1 on H2O2-induced apoptosis in RPTC using siRNA specific for TGase-1. As shown in Fig. 1, D and E, TGase-1 siRNA transfection increased H2O2-induced apoptosis and decreased cell viability. RPTC transfected with TGase-1-siRNA reduced the expression level of TGase-1, while transfection of scrambled siRNA did not affect TGase-1 expression (Fig. 2, E and F). Taken together, these data indicate that exposure of RPTC to H2O2 results in TGase-1 activation, which is required for cell survival under oxidant stress.
Overexpression of TGase-1 promotes RPTC survival following H2O2 exposure.
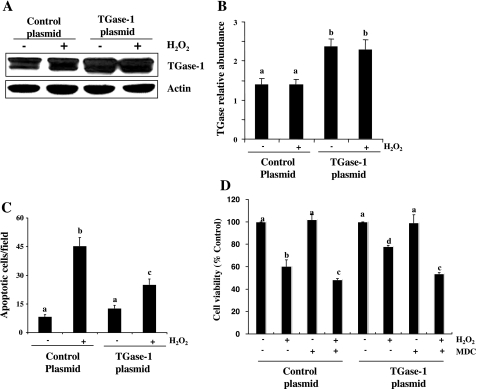
To confirm the role of TGase-1 in RPTC survival, we further examined the effect of overexpression of TGase-1 on H2O2-induced cell death. Figure 3, A and B, show that the TGase-1 expression level was increased up to twofold in RPTC transfected with TGase-1 plasmid compared with cells transfected with an empty vector. Cell viability was increased in H2O2 treated RPTC transfected with TGase-1. Conversely, H2O2-induced apoptosis was suppressed by TGase-1 overexpression (Fig. 3, C and D). In the presence of MDC, TGase-1 overexpression conferred survival advantage to RPTC was completely blocked. As expected, MDC also decreased cell viability in RPTC overexpressing empty vector following H2O2 exposure (Fig. 3D). There was no difference in the basal level of apoptosis in RPTC transfected with either TGase-1 or empty vector alone. Taken together, these data support the role of TGase-1 as a survival molecule in H2O2-induced apoptosis.
Fig. 3.
Effect of TGase-1 overexpression on H2O2-induced cell death in RPTC. RPTC were transiently transfected with plasmids encoding wild-type TGase-1 and then starved for 24 h before treatment with 1 mM H2O2 for 30 min (A) or 4 h in the absence (C and D) or presence of MDC (D). Cell lysates were subjected to immunoblot analysis with antibodies for TGase-1 and actin (A). Representative immunoblots from 3 experiments are shown. The level of TGase-1 was quantified by densitometry and normalized to actin level (B). Apoptosis was evaluated by DAPI staining (C). Cell viability was assessed by MTT assay (D). Values are means ± SD of 3 independent experiments conducted in triplicates and expressed as total apoptotic cells per field (C) or the percentage of cells treated with empty plasmid (D). Bars with different letters are significantly different from one another (P < 0.05).
TGase-1 induces AKT and STAT3 activation following oxidant injury.
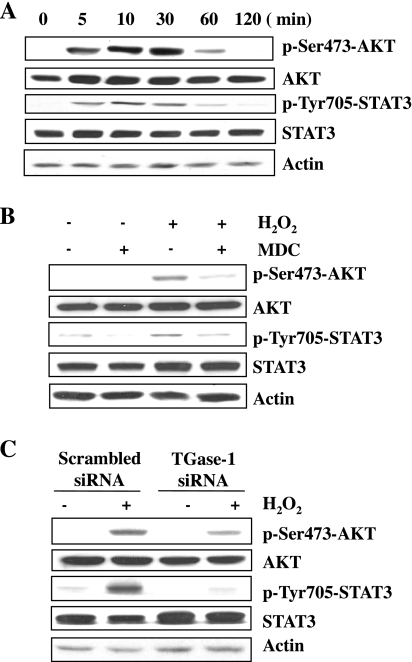
Numerous studies (25, 26, 31, 41) indicate that activation of AKT and STAT3 plays a central role in cell survival against a variety of stress stimuli, including H2O2. We examined whether these two pathways play a role in transducing TGase-1-mediated survival of RPTC. Exposure of RPTC to H2O2 induced the phosphorylation of AKT and STAT3 in a time-dependent manner, which was detected within 5 min, reached maximum at 30 min, and was sustained for 60 min. Total expression levels of AKT and STAT3 did not change (Fig. 4A). H2O2-induced phosphorylation of AKT and STAT3 was suppressed by MDC (Fig. 4B) or TGase-1 siRNA (Fig. 4C). These data indicate that TGase-1 mediates activation of AKT and STAT3 in RPTC following oxidant injury.
Fig. 4.
TGase-1 is required for H2O2-induced activation of AKT and signal transducer and activator of transcription-3 (STAT3). RPTC were exposed to 1mM H2O2 for indicated time (A) or incubated with MDC for 1 h and treated with 1 mM H2O2 for 30 min (B). RPTC cells were transfected with scrambled siRNA or TGase-1-specific siRNA, starved for 24 h, and then treated with 1 mM H2O2 for 30 min (C). Cell lysates were prepared and subjected to immunoblot analysis with antibodies for p-Ser473-AKT, AKT, p-Tyr705-STAT3, STAT3, or actin. Representative immunoblots from 3 experiments are shown.
Activation of AKT and STAT3 is required for RPTC survival following oxidant injury.
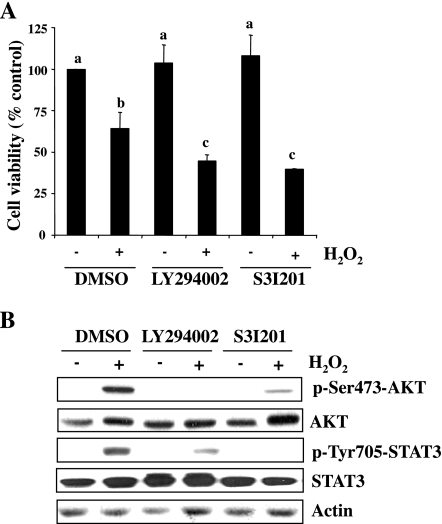
To elucidate the role of AKT and STAT3 in RPTC survival following oxidant injury, we pretreated RPTC with LY294002, an inhibitor for PI3K (the direct upstream activator of AKT; Ref. 45), and S3I201, a STAT3-specific inhibitor (40), and then exposed them to H2O2. Cell viability was measured by the MTT assay. As shown in Fig. 5A, treatment with either LY294002 or S3I201 enhanced H2O2-induced apoptotic cell death. As expected, these two inhibitors abolished phosphorylation of AKT and STAT3, respectively. Interestingly, LY294002 treatment also partially reduced STAT3 phosphorylation and S3I201 reduced AKT phosphorylation in RPTC exposed to H2O2. The loading protein was similar in each sample as shown by immunoblot analysis of actin (Fig. 5B). These data indicate that activation of STAT3 and AKT pathways is required for cell survival in H2O2-treated RPTC and suggest that these two pathways are reciprocally regulated in RPTC under the oxidative stress condition.
Fig. 5.
Effects of AKT and STAT3 inhibition on cell survival following H2O2 expsosure. A: RPTC were incubated with LY294002 (20 μM) or S3I201 (50 μM) for 1 h and then exposed to 1 mM H2O2 for 4 h (A) or 30 min (B). Cell viability was determined by MTT assay. Values are means ± SD of 3 independent experiments conducted in triplicates and expressed as percentage of control. Bars with different letters are significantly different from one another (P < 0.05; A). Cell lysates were subjected to immunoblot with antibodies for p-Ser473-AKT, AKT, p-Tyr705-STAT3, STAT3, or actin. Representative immunoblots from 3 experiments are shown in B.
Downregulation of STAT3 enhances H2O2-induced cell death and inhibition of AKT phosphorylation.
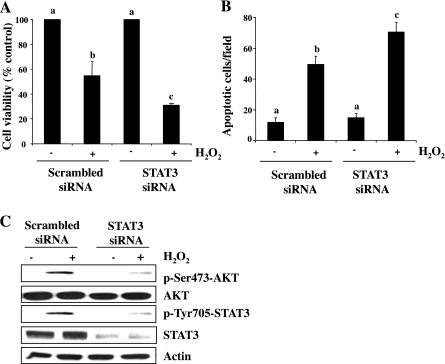
To confirm the involvement of STAT3 in RPTC survival and AKT phosphorylation following oxidant injury, we further examined the effect of STAT3 siRNA on these responses. As shown in Fig. 6, A and B, transfection of STAT3 siRNA enhanced the H2O2-induced apoptotic cell death, as indicated by decreased cell viability and increased apoptotic cells. Consistent with the inhibitory effect of S3I201 on AKT phosphorylation, STAT3-siRNA transfection also reduced H2O2-induced phosphorylation of AKT (Fig. 6C), implicating that STAT3 is indeed required for cell survival and activation of AKT in RPTC following oxidant injury.
Fig. 6.
Effect of STAT3 specific siRNA on H2O2-induced cell death. RPTC were transfected with scrambled siRNA or siRNA specific for STAT3, starved for 24 h, and then treated with 1 mM H2O2 for 4 h (A and B) or for 30 min (C). Cell viability and apoptosis were assessed by MTT assay (A) and DAPI staining (B), respectively. Cell lysates were prepared for immunoblot analysis using antibodies against p-Ser473-AKT, AKT, p-Tyr705-STAT3, STAT3, or actin (C). Values are means ± SD of 3 independent experiments conducted in triplicates and expressed aspercentage of cells treated with scramble siRNA or total number. Bars with different letters are significantly different from one another (P < 0.05). Representative immunoblots from 3 experiments are shown.
Blockade of the PI3K/AKT and STAT3 pathways inhibits the TGase-1 overexpression-induced cytoprotection in RPTC following oxidant injury.
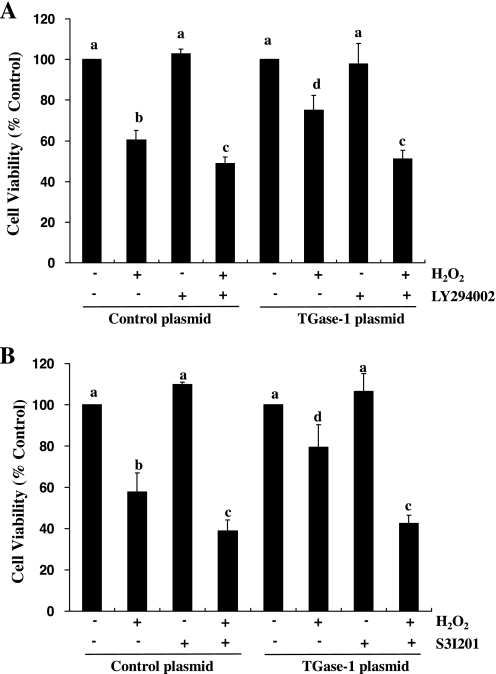
To further determine whether PI3K/AKT and STAT3 pathways transduces TGase-1-mediated cell survival, we examined the effect of inhibition of the PI3K/AKT and STAT3 pathways on cell survival in RPTC overexpressing TGase-1. As shown in Fig. 7, treatment of cells with either LY294002 or S3I201 abolished the enhancement of cell survival in RPTC overexpressing TGase-1 following oxidant injury. These data further support our conclusions that TGase-1 mediates cell survival through activation of the PI3K/AKT and STAT3 signaling pathways.
Fig. 7.
Effect of LY294002 (A) and S3I201 (B) on cell survival following H2O2 in RPTC overexpressing TGase-1. RPTC were transfected with control plasmid or plasmid encoding wild-type TGase-1, starved for 24 h, and then treated with 1 mM H2O2 for 4 h in the absence or presence of LY29400 (20 μM) or S3I201(50 μM). Cell viability was assessed by MTT assay. Values are means ± SD of 3 independent experiments conducted in triplicates and expressed as the percentage of control. Bars with different letters are significantly different from one another (P < 0.05).
TGase-1 mediates inactivation of GSK-3β following oxidant injury.
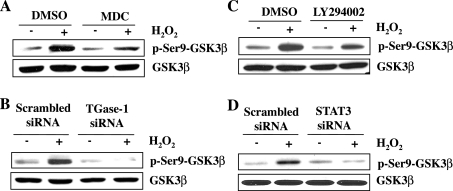
GSK-3β activation is associated with cytochrome c release and apoptotic cell death in a variety of cell types in response to oxidant injury (9, 24). AKT can induce its inactivation by direct phosphorylation at serine 9 (24). Since the above data revealed that TGase-1 mediated AKT activation following oxidant injury, it is possible that TGase-1 would also regulate GSK-3β activity. To test this hypothesis, we examined the effect of TGase-1 inhibition on phosphorylation of GSK-3β at serine 9. GSK-3β is constitutively activated, and its phosphorylation at serine 9 is inactive. As shown in Fig. 8, A and B, treatment of RPTC with either MDC or TGase-1 siRNA decreased H2O2-induced GSK-3β phosphorylation at serine 9. Inhibition of AKT with LY 294002 and downregulation of STAT3 with siRNA also inhibited GSK-3β phosphorylation (Fig. 8, C and D). These results suggest that TGase-1 may inactivate GSK-3β through activation of AKT and STAT3 in RPTC treated with H2O2.
Fig. 8.
Effects of inhibition of TGase-1, phosphoinositide 3-kinase (PI3K)/AKT, or STAT3 on GSK-3β phosphorylation following oxidant injury. Serum-starved RPTC were pretreated with 100 μM MDC (A) and 20 μM LY294002 (C) for 1 h and then treated with 1 mM H2O2 for 30 min. RPTC were transfected with scrambled siRNA or TGase-1 siRNA (B) or STAT3 siRNA (D), starved for 24 h, and then exposed to 1 mM H2O2 for 30 min. Cell lysates were subjected to immunoblot analysis using antibodies against p-Ser9-GSK-3β, GSK-3β, or actin. Representative immunoblots from 3 experiments are shown.
Inactivation of GSK-3β confers survival to RPTC and abolishes the death-promoting effect of AKT and STAT3 inhibition following oxidant injury.
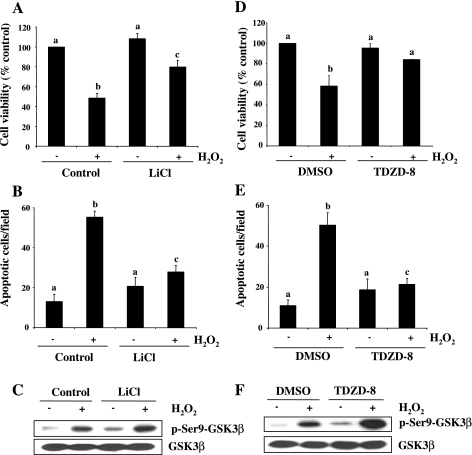
It has been reported that GSK-3β activation either contributes to cell death or promotes cell survival, depending on cell types and stimulation (9, 10). To determine the role of GSK-3β activation in RPTC apoptosis following oxidant injury, we examined the effect of inhibition of GSK-3β on H2O2-induced apoptosis using lithium chloride (LiCl) or TDZD-8, two selective GSK-3β inhibitors that induce phosphorylation of GSK-3β at serine 9 and inactivate them (21, 42). Inactivation of GSK-3β by LiCl increased the RPTC viability (Fig. 9A) and decreased the apoptotic cell death (Fig. 9B). Similar results were obtained when cells were treated with TDZD-8 (Fig. 9, D and E). Treatment with LiCl or TDZD-8 slightly increased the basal level of GSK-3β phosphorylation and enhanced H2O2-induced GSK-3β phosphorylation (Fig. 9, C and F). These results suggest that GSK-3β activation contributes to apoptosis in RPTC following oxidant injury.
Fig. 9.
Effect of GSK-3β inactivation on RPTC survival following oxidant injury. Serum-starved RPTC were pretreated with LiCl (20 mM) and TDZD-8 (40 μM) for 1 h and then exposed to 1 mM H2O2 for 4 h (A, B, D, and E) or for 30 min (C and F). Cell viability and apoptosis were assessed by the MTT assay (A and D) and DAPI staining (B and E), respectively. Values are means ± SD of 3 independent experiments conducted in triplicates and expressed as the percentage of control (A and D) or total apoptotic cells per field (B and E). Bars with different letters are significantly different from one another (P < 0.05). Cell lysates were prepared for immunoblot analysis using antibodies against p-Ser9-GSK-3β and GSK-3β (C and F). Representative immunoblots from 3 experiments are shown.
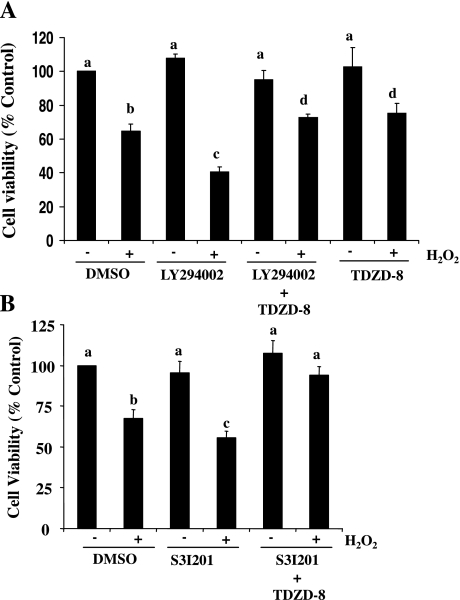
The above data (Figs. 5–8) show that blockade of either the PI3K/AKT or STAT3 pathway potentiates cell death and inactivates GSK-3β by phosphorylation at serine 9, suggesting that activation of the PI3K/AKT and STAT3 pathways may contribute to cell survival through inactivation of GSK-3β. If this is indeed the case, inactivation of GSK-3β should block the death-promoting effect of AKT and STAT3 inhibition. To test this hypothesis, RPTC were treated with the PI3K/Akt pathway inhibitor (LY294002) or STAT3 inhibitor (S3I201) in the absence or presence of TDZD-8 before H2O2 exposure. As shown in Fig. 10, TDZD-8 treatment abolished the inhibitory effect of LY294002 and S3I201 on cell survival under oxidant stress. Similar results were obtained when RPTC overexpressing TGase-1 were treated with those inhibitors (data not shown). These data, together with the inhibitory effect of MDC and TGase-1 siRNA on GSK-3β phosphorylation (Fig. 8), suggest that TGase-1 induces cell survival through the AKT/STAT3/GSK-3β pathway in RPTC after oxidant injury.
Fig. 10.
TDZD-8 treatment abolished the death-promoting effect of LY294002 and S3I201 in RPTC following oxidant injury. RPTC were treated with 1 mM H2O2 for 4 h in the absence or presence of LY29400 (20 μM) or S3I201 (50 μM) with/without TDZD-8 (40 μM). Cell viability was assessed by the MTT assay. Values are means ± SD of 3 independent experiments conducted in triplicates and expressed as the percentage of control. Bars with different letters are significantly different from one another (P < 0.05).
DISCUSSION
ROS including H2O2 are generated following I/R and toxicant exposure and are critically involved in the pathogenesis of AKI (5, 7, 22). In this study, we demonstrated that the exposure of RPTC to H2O2 increased TGase activation and induced apoptosis. Inhibition of TGase activity by a pharmacological inhibitor (MDC) and reduction of TGase-1 expression with siRNA potentiated H2O2-induced apoptotic cell death. Conversely, overexpression of TGase-1 inhibited the apoptosis and increased the cell viability. Therefore, we have identified the novel function of TGase-1 as a survival factor in renal epithelial cells and its activation protects RPTC from apoptosis following oxidant injury.
The ability of cells to survive a variety of stresses including oxidant stress often depends on the activation of survival signaling pathways. PI3K/AKT and JAK2-STAT3 pathways have been shown to be activated by H2O2 (15, 32, 37, 41) and mediate cell survival in a variety of cell types including renal epithelial cells (2, 8, 17, 31). In this study, we observed that H2O2-induced phosphorylation of AKT and STAT3 was inhibited by either the selective TGase inhibitor MDC or siRNA specifically targeting TGase-1. Furthermore, inhibition of either the AKT or STAT3 signaling pathway potentiated apoptosis and abolished TGase-1 overexpression, inducing the cytoprotective effect in RPTC exposed to H2O2. Therefore, we suggest that the PI3K/AKT and STAT3 pathways mediate the prosurvival function of TGase-1 in renal epithelial cells. The mechanism coupling TGase-1 to the activation of AKT and STAT3 remains unclear. Verma et al. (43, 44) reported that overexpression of TGase-2 can induce inactivation of PTEN, a negative regulator of the PI3K/AKT signaling pathway in pancreatic ductal adenocarcinoma cells. Our previous studies (49) showed that TGase-1 promotes JAK2-mediated phosphorylation of STAT3 in serum-stimulated RPTC. Therefore, TGase-1 may mediate activation of AKT and STAT3 through PTEN- and JAK2-dependent mechanisms, respectively. In addition, since JAK2 has been reported to induce phosphorylation of AKT via activation of PI3K (1, 33, 47), TGase-1 may also induce AKT activation via targeting JAK2 in RPTC.
We noticed that the time course for activation of TGase-1 and phosphorylation of STAT3 and AKT was not exactly the same in RPTC following H2O2 exposure: while TGase activity was maximal at 2 h, AKT and STAT3 phosphorylation was maximal at 30 min. However, both TGase-1 activity and STAT3 and AKT phosphorylation were detected within 10 min after stimulation with H2O2. These data, together with our observations that inhibition of TGase activity with MDC reduced phosphorylation of AKT and STAT3, suggest that the early increase in TGase-1 activity is sufficient to cause their activation. A possible explanation for the inconsistent time courses of AKT/STAT3 phosphorylation and TGase-1 activity is that phosphorylation of AKT and STAT3 is balanced by activation of kinases and phosphatases. A rapid decline in AKT and STAT3 phosphorylation after oxidant injury may be due to a stronger activation of the phosphatases that mediate dephosphorylation of these kinases or their upstream activators. In support of this hypothesis, it has been reported that SHP-2, a phosphatase of both Akt and STAT3 (16, 27, 48), can be activated within 5 min and reduces STAT3 tyrosine phosphorylation level in response to oxidant stress (34). Therefore, while our data support the role of TGase-1 in regulating AKT and STAT3 activation, a causal link and the detailed mechanism for TGase-1 to activate these two pathways need further investigation.
Our data suggest that AKT and STAT3 transduce the survival signal of TGase-1 through inactivation of GSK-3β in H2O2-treated RPTC. Previous studies (24) have shown that AKT mediates cell survival via phosphorylation of GSK-3β at serine 9 (inactivation of GSK-3β), and our current study demonstrated that inhibition of the PI3K/AKT pathway with LY294002 blocked GSK-3β phosphorylation in H2O2-treated RPTC. As such, if TGase-1 functions upstream of AKT to mediate cell survival, inhibition of TGase-1 should also inhibit GSK-3β phosphorylation at serine 9. Indeed, our data indicate that inhibition of TGase-1 with MDC or siRNA abolished H2O2-induced phosphorylation of GSK-3β at this residue. Additionally, inactivation of STAT3 also inhibited H2O2-induced GSK-3β phosphorylation, implying that GSK-3β is also subjected to regulation by STAT3. These results, together with the necessity of TGase-1 in mediating phosphorylation of AKT and STAT3, suggest that TGase-1 protects RPTC against apoptosis through PI3K/AKT- and STAT3-mediated inactivation of GSK-3β. In support of this conclusion, we further demonstrated that inactivation of GSK-3β abolished the death-promoting effect of AKT and STAT3 inhibition in RPTC overexpressing TGase-1 after oxidant injury.
Interestingly, a cross talk between the PI3K/AKT and JAK/STAT3 signaling pathways exists in RPTC exposed to oxidant injury. This statement was supported by our observations that inhibition of AKT activation by LY294002, a specific PI3K inhibitor, decreased STAT3 phosphorylation, and vice versa inhibition of STAT3 activation by S3I201 or knockdown of STAT3 with specific siRNA targeting STAT3, reduced AKT phosphorylation in H2O2-treated RPTC. In line with our observations, previous studies (2, 23, 38) reveal the potential cross talk between the PI3K/AKT and JAK/STAT3 signaling pathways in other cell types. For example, STAT3 can function as an adaptor molecule that promotes PI3K activation, and exposure of H2O2 triggers activation of PI3K (38). As such, a decrease in AKT phosphorylation upon STAT3 inhibition observed in our study is likely the result of decreased activity of PI3K and/or inactivation of its downstream signaling molecules. While it is unclear whether PI3K and AKT can directly regulate STAT3 activation, a recent study (9) shows that STAT3 activation induced by interferon-γ is highly dependent on the activity of GSK-3β in mouse primary astrocytes, microglia, and macrophage-derived RAW264.7 cells. In this study, we examined the possible role of GSK-3β in H2O2-induced STAT3 activation; however, inhibition of GSK-3β with either LiCl or TDZD-8 did not alter the phosphorylation level of STAT3 (data not shown), suggesting that GSK-3β does not mediate activation of STAT3 under oxidant stress conditions. Despite the importance of the cross talk between these two signaling pathways in providing a signal for renal epithelial cells resistant to apoptosis following oxidant injury, blockade of the STAT3 or the AKT pathway only partially inhibits each other's phosphorylation, suggesting that other specific signaling molecules may also contribute to their activation. Further investigations are needed to identify the signaling molecules that are specifically coupled to those signaling pathways.
Although TGase-1 activity is required for RPTC survival, the mechanisms that regulate TGase-1 activation following oxidant injury remain to be defined. It has been reported that activation of epidermal growth factor receptor (EGFR) by epidermal growth factor resulted in a potent increase in TGase activity in breast cancer cells and that the PI3K/AKT pathway mediates the action of EGFR in this process (3). Our recent studies (50) have shown that exposure of RPTC to H2O2 can induce EGFR activation, which in turn activates AKT, suggesting that the EGFR-PI3K/AKT pathway may be involved in regulating the activation of TGase-1 in renal epithelial cells. These studies, together with our observation that TGase-1-mediated activation of AKT is required for RPTC survival, suggest that the PI3K/AKT pathway may act both upstream and downstream of TGase-1 in regulating cell survival following oxidant injury. Elucidating the signaling events responsible for EGFR-mediated TGase activation is a focus of our ongoing investigations.
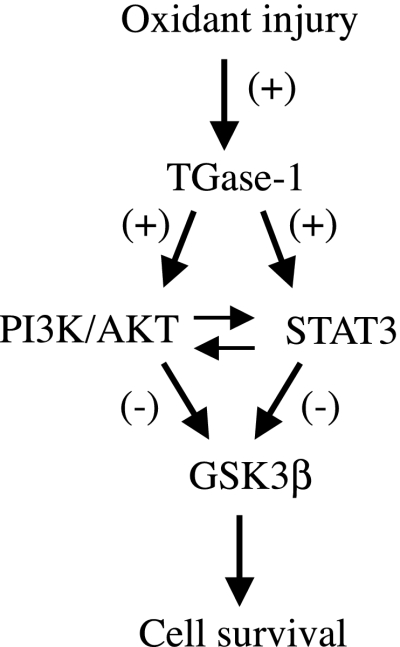
Taken together, our studies demonstrate that after oxidant injury, TGase-1 is activated, which in turn stimulates activation of AKT and STAT3 and subsequent inactivation of GSK-3β Furthermore, a cross talk exists between the PI3K/AKT and STAT3 pathways (Fig. 11) and TGase-1-mediated activation of the PI3K/AKT and STAT3 pathway is required for RPTC survival following oxidant injury. Since oxidant injury-induced renal epithelial cell death is critically involved in the development of AKI, this study provides a new insight for the pathophysiology of acute renal failure.
Fig. 11.
Schematic illustration of TGase-1-induced signaling pathways leading to RPTC survival following oxidant injury. TGase-1 is activated and required for RPTC survival following H2O2 exposure. The survival signal of TGase-1 is transduced by PI3K/AKT and STAT3, which in turn inhibits the activity of GSK-3β.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-071997.
REFERENCES
- 1.Al-Shami A, Naccache PH. Granulocyte-macrophage colony-stimulating factor-activated signaling pathways in human neutrophils. Involvement of Jak2 in the stimulation of phosphatidylinositol 3-kinase. J Biol Chem 274: 5333–5338, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Al Zaid Siddiquee K, Turkson J. STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell Res 18: 254–267, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonyak MA, Miller AM, Jansen JM, Boehm JE, Balkman CE, Wakshlag JJ, Page RL, Cerione RA. Augmentation of tissue transglutaminase expression and activation by epidermal growth factor inhibit doxorubicin-induced apoptosis in human breast cancer cells. J Biol Chem 279: 41461–41467, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Antonyak MA, Singh US, Lee DA, Boehm JE, Combs C, Zgola MM, Page RL, Cerione RA. Effects of tissue transglutaminase on retinoic acid-induced cellular differentiation and protection against apoptosis. J Biol Chem 276: 33582–33587, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Aragno M, Cutrin JC, Mastrocola R, Perrelli MG, Restivo F, Poli G, Danni O, Boccuzzi G. Oxidative stress and kidney dysfunction due to ischemia/reperfusion in rat: attenuation by dehydroepiandrosterone. Kidney Int 64: 836–843, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Bae J, Lee YS, Jeoung D. Downregulation of transglutaminase II leads to impaired motility of cancer cells by inactivation of the protein kinase, Akt, and decrease of reactive oxygen species. Biotechnol Lett 28: 1151–1158, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Basnakian AG, Kaushal GP, Shah SV. Apoptotic pathways of oxidative damage to renal tubular epithelial cells. Antioxid Redox Signal 4: 915–924, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Battle TE, Frank DA. The role of STATs in apoptosis. Curr Mol Med 2: 381–392, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Beurel E, Jope RS. Differential regulation of STAT family members by glycogen synthase kinase-3. J Biol Chem 283: 21934–21944, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beurel E, Jope RS. The paradoxical pro- and anti-apoptotic actions of GSK-3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol 79: 173–189, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boehm JE, Singh U, Combs C, Antonyak MA, Cerione RA. Tissue transglutaminase protects against apoptosis by modifying the tumor suppressor protein p110 Rb. J Biol Chem 277: 20127–20130, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Cao L, Petrusca DN, Satpathy M, Nakshatri H, Petrache I, Matei D. Tissue transglutaminase protects epithelial ovarian cancer cells from cisplatin-induced apoptosis by promoting cell survival signaling. Carcinogenesis 29: 1893–1900, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Datta S, Antonyak MA, Cerione RA. Importance of Ca(2+)-dependent transamidation activity in the protection afforded by tissue transglutaminase against doxorubicin-induced apoptosis. Biochemistry 45: 13163–13174, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ernest S, Bello-Reuss E. Expression and function of P-glycoprotein in a mouse kidney cell line. Am J Physiol Cell Physiol 269: C323–C333, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Hagiwara M, Shen B, Chao L, Chao J. Kallikrein-modified mesenchymal stem cell implantation provides enhanced protection against acute ischemic kidney injury by inhibiting apoptosis and inflammation. Hum Gene Ther 19: 807–819, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haider UG, Roos TU, Kontaridis MI, Neel BG, Sorescu D, Griendling KK, Vollmar AM, Dirsch VM. Resveratrol inhibits angiotensin II- and epidermal growth factor-mediated Akt activation: role of Gab1 and Shp2. Mol Pharmacol 68: 41–48, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Harris TK. PDK1 and PKB/Akt: ideal targets for development of new strategies to structure-based drug design. IUBMB Life 55: 117–126, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Hiiragi T, Sasaki H, Nagafuchi A, Sabe H, Shen SC, Matsuki M, Yamanishi K, Tsukita S. Transglutaminase type 1 and its cross-linking activity are concentrated at adherens junctions in simple epithelial cells. J Biol Chem 274: 34148–34154, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Ientile R, Caccamo D, Griffin M. Tissue transglutaminase and the stress response. Amino Acids 33: 385–394, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Kelly KJ, Molitoris BA. Acute renal failure in the new millennium: time to consider combination therapy. Semin Nephrol 20: 4–19, 2000 [PubMed] [Google Scholar]
- 21.Kim SD, Yang SI, Kim HC, Shin CY, Ko KH. Inhibition of GSK-3beta mediates expression of MMP-9 through ERK1/2 activation and translocation of NF-kappaB in rat primary astrocyte. Brain Res 1186: 12–20, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Koyner JL, Sher Ali R, Murray PT. Antioxidants. Do they have a place in the prevention or therapy of acute kidney injury? Nephron Exp Nephrol 109: e109–117, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Krasilnikov M, Ivanov VN, Dong J, Ronai Z. ERK and PI3K negatively regulate STAT-transcriptional activities in human melanoma cells: implications towards sensitization to apoptosis. Oncogene 22: 4092–4101, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Lin CF, Chen CL, Chiang CW, Jan MS, Huang WC, Lin YS. GSK-3beta acts downstream of PP2A and the PI 3-kinase-Akt pathway, and upstream of caspase-2 in ceramide-induced mitochondrial apoptosis. J Cell Sci 120: 2935–2943, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Lu Y, Zhou J, Xu C, Lin H, Xiao J, Wang Z, Yang B. JAK/STAT and PI3K/AKT pathways form a mutual transactivation loop and afford resistance to oxidative stress-induced apoptosis in cardiomyocytes. Cell Physiol Biochem 21: 305–314, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Madamanchi NR, Li S, Patterson C, Runge MS. Reactive oxygen species regulate heat-shock protein 70 via the JAK/STAT pathway. Arterioscler Thromb Vasc Biol 21: 321–326, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Mattoon DR, Lamothe B, Lax I, Schlessinger J. The docking protein Gab1 is the primary mediator of EGF-stimulated activation of the PI-3K/Akt cell survival pathway. BMC Biol 2: 24, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mishra S, Melino G, Murphy LJ. Transglutaminase 2 kinase activity facilitates protein kinase A-induced phosphorylation of retinoblastoma protein. J Biol Chem 282: 18108–18115, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Mishra S, Murphy LJ. The p53 oncoprotein is a substrate for tissue transglutaminase kinase activity. Biochem Biophys Res Commun 339: 726–730, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Mishra S, Murphy LJ. Tissue transglutaminase has intrinsic kinase activity: identification of transglutaminase 2 as an insulin-like growth factor-binding protein-3 kinase. J Biol Chem 279: 23863–23868, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Mullonkal CJ, Toledo-Pereyra LH. Akt in ischemia and reperfusion. J Invest Surg 20: 195–203, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Nair VD, Olanow CW. Differential modulation of Akt/glycogen synthase kinase-3beta pathway regulates apoptotic and cytoprotective signaling responses. J Biol Chem 283: 15469–15478, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogunwobi OO, Beales IL. Glycine-extended gastrin stimulates proliferation via JAK2- and Akt-dependent NF-kappaB activation in Barrett's oesophageal adenocarcinoma cells. Mol Cell Endocrinol 296: 94–102, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Park SJ, Kim HY, Kim H, Park SM, Joe EH, Jou I, Choi YH. Oxidative stress induces lipid-raft-mediated activation of Src homology 2 domain-containing protein-tyrosine phosphatase 2 in astrocytes. Free Radic Biol Med 46: 1694–1702, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Perry MJ, Mahoney SA, Haynes LW. Transglutaminase C in cerebellar granule neurons: regulation and localization of substrate cross-linking. Neuroscience 65: 1063–1076, 1995 [DOI] [PubMed] [Google Scholar]
- 36.Rana A, Sathyanarayana P, Lieberthal W. Role of apoptosis of renal tubular cells in acute renal failure: therapeutic implications. Apoptosis 6: 83–102, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Sadidi M, Lentz SI, Feldman EL. Hydrogen peroxide-induced Akt phosphorylation regulates Bax activation. Biochimie 91: 577–585, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schweizer U, Gunnersen J, Karch C, Wiese S, Holtmann B, Takeda K, Akira S, Sendtner M. Conditional gene ablation of Stat3 reveals differential signaling requirements for survival of motoneurons during development and after nerve injury in the adult. J Cell Biol 156: 287–297, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheridan AM, Bonventre JV. Cell biology and molecular mechanisms of injury in ischemic acute renal failure. Curr Opin Nephrol Hypertens 9: 427–434, 2000 [DOI] [PubMed] [Google Scholar]
- 39a.Shin DM, Jeon JH, Kim CW, Cho SY, Kwon JC, Lee HJ, Choi KH, Park SC, Kim IG. Cell type specific activation of intracellular transglutaminase 2 by oxidative stress or ultraviolet irradiation: implications of transglutaminase 2 in age-related cataractogenesis. J Biol Chem 279: 15032–15039, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Siddiquee K, Zhang S, Guida WC, Blaskovich MA, Greedy B, Lawrence HR, Yip ML, Jove R, McLaughlin MM, Lawrence NJ, Sebti SM, Turkson J. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci USA 104: 7391–7396, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simon AR, Rai U, Fanburg BL, Cochran BH. Activation of the JAK-STAT pathway by reactive oxygen species. Am J Physiol Cell Physiol 275: C1640–C1652, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol 6: 1664–1668, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Verma A, Guha S, Wang H, Fok JY, Koul D, Abbruzzese J, Mehta K. Tissue transglutaminase regulates focal adhesion kinase/AKT activation by modulating PTEN expression in pancreatic cancer cells. Clin Cancer Res 14: 1997–2005, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Verma A, Wang H, Manavathi B, Fok JY, Mann AP, Kumar R, Mehta K. Increased expression of tissue transglutaminase in pancreatic ductal adenocarcinoma and its implications in drug resistance and metastasis. Cancer Res 66: 10525–10533, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem 269: 5241–5248, 1994 [PubMed] [Google Scholar]
- 46.Yamaguchi H, Wang HG. Tissue transglutaminase serves as an inhibitor of apoptosis by cross-linking caspase 3 in thapsigargin-treated cells. Mol Cell Biol 26: 569–579, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamauchi T, Kaburagi Y, Ueki K, Tsuji Y, Stark GR, Kerr IM, Tsushima T, Akanuma Y, Komuro I, Tobe K, Yazaki Y, Kadowaki T. Growth hormone and prolactin stimulate tyrosine phosphorylation of insulin receptor substrate-1, -2, and -3, their association with p85 phosphatidylinositol 3-kinase (PI3-kinase), and concomitantly PI3-kinase activation via JAK2 kinase. J Biol Chem 273: 15719–15726, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Zhang SQ, Tsiaras WG, Araki T, Wen G, Minichiello L, Klein R, Neel BG. Receptor-specific regulation of phosphatidylinositol 3′-kinase activation by the protein tyrosine phosphatase Shp2. Mol Cell Biol 22: 4062–4072, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Z, Xing J, Ma L, Gong R, Chin YE, Zhuang S. Transglutaminase-1 regulates renal epithelial cell proliferation through activation of Stat-3. J Biol Chem 284: 3345–3353, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhuang S, Schnellmann RG. H2O2-induced transactivation of EGF receptor requires Src and mediates ERK1/2, but not Akt, activation in renal cells. Am J Physiol Renal Physiol 286: F858–F865, 2004 [DOI] [PubMed] [Google Scholar]