Abstract
Oxidative stress with hydrogen peroxide (H2O2) readily promotes early afterdepolarizations (EADs) and triggered activity (TA) in isolated rat and rabbit ventricular myocytes. Here we examined the effects of H2O2 on arrhythmias in intact Langendorff rat and rabbit hearts using dual-membrane voltage and intracellular calcium optical mapping and glass microelectrode recordings. Young adult rat (3–5 mo, N = 25) and rabbit (3–5 mo, N = 6) hearts exhibited no arrhythmias when perfused with H2O2 (0.1–2 mM) for up to 3 h. However, in 33 out of 35 (94%) aged (24–26 mo) rat hearts, 0.1 mM H2O2 caused EAD-mediated TA, leading to ventricular tachycardia (VT) and fibrillation (VF). Aged rabbits (life span, 8–12 yr) were not available, but 4 of 10 middle-aged rabbits (3–5 yr) developed EADs, TA, VT, and VF. These arrhythmias were suppressed by the reducing agent N-acetylcysteine (2 mM) and CaMKII inhibitor KN-93 (1 μM) but not by its inactive form (KN-92, 1 μM). There were no significant differences between action potential duration (APD) or APD restitution slope before or after H2O2 in aged or young adult rat hearts. In histological sections, however, trichrome staining revealed that aged rat hearts exhibited extensive fibrosis, ranging from 10–90%; middle-aged rabbit hearts had less fibrosis (5–35%), whereas young adult rat and rabbit hearts had <4% fibrosis. In aged rat hearts, EADs and TA arose most frequently (70%) from the left ventricular base where fibrosis was intermediate (∼30%). Computer simulations in two-dimensional tissue incorporating variable degrees of fibrosis showed that intermediate (but not mild or severe) fibrosis promoted EADs and TA. We conclude that in aged ventricles exposed to oxidative stress, fibrosis facilitates the ability of cellular EADs to emerge and generate TA, VT, and VF at the tissue level.
Keywords: optical mapping, aging, fibrosis, calcium transient, triggered activity, early afterdepolarization
hydrogen peroxide (H2O2) readily promotes early afterdepolarizations (EADs) and triggered activity (TA) in isolated rat and rabbit ventricular myocytes (32, 36). In tissue, however, EADs may be suppressed by source-sink mismatches arising from cell-to-cell coupling; that is, a small current that is sufficient to reverse repolarization and cause an EAD in an isolated myocyte will be diluted into adjacent repolarizing myocytes (unless they are also simultaneously primed for an EAD), thereby suppressing the EAD. We investigated this issue by examining the effects of H2O2 on arrhythmias in Langendorff rat and rabbit hearts, using H2O2 concentrations that readily induce EADs in isolated myocytes from these species. Consistent with the predicted suppressive effects of well-coupled tissue on EADs, we found that oxidative stress with H2O2 failed to induce any ventricular arrhythmias in young adult rat or rabbit hearts. However, in aged rat (24–26 mo, normal rat life span, 30–36 mo) hearts in which cell-to-cell coupling was regionally altered by extensive fibrosis (10–90%), H2O2 exposure consistently caused EADs and TA, leading to ventricular tachycardia (VT) and fibrillation (VF) in >90% of the hearts. Middle-aged rabbit (3–5 yr; normal rabbit life span, 8–12 yr) hearts, in which fibrosis was less extensive (5–35%), developed EADs, TA, VT, and VF in 40% of the hearts. Although other aging-related factors may also play a role, we hypothesize that reduced cell-to-cell coupling in fibrotic aged hearts promotes oxidative EAD-related arrhythmias by favorably altering source-sink relationships. Computer simulations of two-dimensional (2-D) cardiac tissue incorporating fibrosis supported this interpretation.
MATERIALS AND METHODS
The research protocol was approved by the Institutional Animal Care and Use Committee and followed the guidelines of the American Heart Association.
Langendorff Setup
Male Fisher 344 adult (3–5 mo; N = 25) and aged (24–26 mo; N = 35) rats and adult White New Zealand rabbits (N = 6) ages 3–5 mo and middle aged (N = 10) between ages 3 to 5 yr were used in this study. The exact age of these rabbits could not be ascertained. The aged rats were purchased from the National Institute on Ageing and the rabbits from a private breeder. The hearts of both species were isolated and perfused with oxygenated Tyrode solution at 37 ± 0.5°C in a Langendorff setting as we described previously (22).
Optical Mapping
The hearts were stained with RH237 and Rhod-2 AM (Invitrogen Molecular Probes, Carlsbad, CA) for simultaneous dual voltage (V) and intracellular calcium (Cai2+) fluorescent optical imaging, respectively, as described previously (22). Cai2+ transient decay rate constant, τ, was determined by a monoexponential fit during the relaxation. Cytochalasin D (5 μmol/l) was added to the perfusate to inhibit motion (9). Single cell action potentials (APs) were recorded with a roving glass microelectrode from left ventricular (LV) epicardial sites showing focal VT as shown by optical voltage activation maps.
Dynamic AP Duration Restitution
AP duration (APD) was measured at 90% (APD90) and 50% (APD50) repolarization, and APD restitution curves were determined using a dynamic pacing protocol (22) from the base of the heart. In each heart, the APs were recorded from 3–5 different sites located at the base, mid-, and apical region of the LV anterior surface. Results were pooled since no differences in APD and APD restitution were found among the different sites and between the two aged groups. When VFs were initiated, they were terminated within 1 min of onset by electrical shocks delivered through a pair of 5-cm-long coil electrodes in the tissue bath located at both sides of the isolated hearts.
Chemical and Pharmacological Interventions
The isolated hearts were exposed to increasing levels 0.05–2 mM of H2O2. In adult rat and rabbit hearts, raising the H2O2 level from 0.01, 0.05, 0.1, 0.2, 1, and 2 mM failed to induce VT/VF in all eight adult rat and six rabbit hearts. In contrast, however, 0.05 mM H2O2 promoted VF in 50% of aged rat hearts and raising its concentration to 0.1 mM caused VF in 94% of the hearts. In middle-aged rabbit hearts, the VF yield was 40% with 0.1 mM and raising it to 1 mM did not increase the VF. Consequently, in this study, we report our findings on VF using 0.1 mM H2O2 in both species. N-acetylcysteine (NAC) at 2 mmol/l infusion was initiated either before (preventive therapy) or after (suppressive therapy) exposure to H2O2. CaMKII inhibitor KN-93 (Sigma) and its inactive form KN-92 (1 μmol/l; Calbiochem, La Jolla, CA) likewise were used to test for their protective and suppressive effect on H2O2-induced VF as with NAC. All other chemicals were obtained from Sigma-Aldrich.
Endocardial and Midmyocardial Cryoablation
Cryoablation of the endocardial and midmyocardial structures were achieved in aged rats using a 7-cm SurgiFrost cryoablation probe (ATS Medical, Minneapolis, MN). The probe was placed in the LV cavity, and its temperature decreased to −55°C for 3–6 min (9).
Histological Analyses
Percent tissue fibrosis was determined using Masson trichrome stain for collagen as previously described (20). Briefly, with the use of a grid that divided the field of view into 100 squares, the number of collagenous tissue (blue stain) at the 100 intersection points in the grid was scored as 1 (present) or 0 (absent). Results are expressed as the percentage occupied by fibrosis to the total area examined. In few tissue samples, fibroblast receptor discoidin domain receptor 2 was used to estimate a rise in fibroblast density in fibrotic aged hearts (3).
Computer Simulation Studies
A 5-cm-by-2.5-cm 2-D tissue with an ellipse fibrosis region of radius 1.8-cm by 0.9-cm in the center was used to simulate the effect of fibroblasts on EAD-triggered focal activity (see supplemental diagram; note: all supplemental material may be found posted with the online version of this article). In the fibrosis region, fibroblasts were coupled with individual myocytes at densities ranging from 0.5 to 3. We modified the rabbit ventricular myocyte model (18) to satisfy the ionic changes in H2O2 perfusion by doubling the maximum conductance of sodium/calcium exchange current (INCX) (6) and peak L-type calcium current (ICa-L) (39) and by decreasing the Cai2+ transient decline rate by 20% (12) and maintaining 0.04 of the peak sodium current (INa) during the repolarization phase to mimic the late INa.(32, 35, 36).
The membrane voltage (V) of a myocyte (Vm) or a fibroblast (Vf) in the tissue model is governed by the following:
where C is the membrane capacitance of a myocyte (Cm) or a fibroblast (Cf), Iion is the corresponding membrane current (Im or If), n is the number of coupled neighbors (either myocytes or fibroblasts), and Ggapk is the gap junction conductance between a cell (either a myocyte or a fibroblast) and its kth neighbor (either a myocyte or a fibroblast). The size of the myocyte was set to 125 μm × 25 μm × 25 μm, with Cm = 125 pF. Im was formulated from the rabbit ventricular model developed by our group (18, 31). The size of the fibroblast was set to 25 μm × 25 μm × 25 μm. For the membrane current of the fibroblast, we mainly used a passive model (15), i.e.,
where Gf is the membrane conductance and Ef is the resting potential of the fibroblast. We set Cf = 25 pF (16, 33), Gf = 3 nS (15), and Ef =−10 mV (14).
To generate the 2-D tissue model, fibroblasts were randomly inserted between myocytes in the fibrotic region, as illustrated in supplemental Fig. 2. To generate this tissue model, we first generated a mesh with a spatial resolution of 25 μm × 25 μm. A fibroblast accounts for one such box, whereas a myocyte accounts for five boxes in a row. Once a fibroblast-to-myocyte ratio (α) is assigned, then the probability of a box being a fibroblast can be determined as α/(α + 5), and a random number is generated to determine whether the present box is a fibroblast or not. If the box is not a fibroblast, then an additional four consecutive boxes in the row are used to form a myocyte (composed of 5 boxes). This process is repeated until all the boxes are assigned as either fibroblasts or myocytes. The gap junction conductance between myocytes was set to 600 nS when two myocytes were coupled end to end and to 1,000 nS when they were fully overlapped side to side. When two myocytes were partially overlapped side to side, the gap junction conductance was proportional to the overlap. This gives rise to a longitudinal conduction velocity of 0.56 m/s and a transverse conduction velocity of 0.13 m/s when no fibroblasts are present. The gap junction conductance between myocyte and fibroblast or between two fibroblasts was set to 5.4 nS (27).
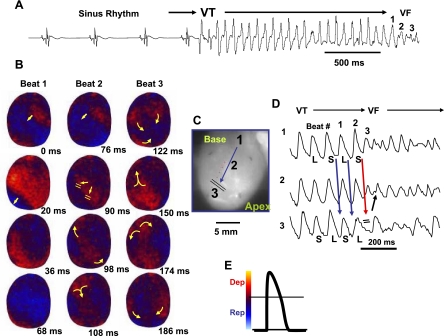
Fig. 2.
Spontaneous initiation of ventricular tachycardia (VT)/VF in an aged rat heart exposed to 0.1 mM H2O2. A: ECG showing the last 5 sinus beats before the sudden onset of VT leading to VF. B: voltage snapshots of the last beat of the VT (beat 1) and of the first 2 beats of the VF (beats 2 and 3). In each snapshot, activation time (in ms) is shown at the bottom right with time 0 (arbitrary) coinciding with the onset of beat 1. The red color in the snapshots represents depolarization (Dep) and the blue repolarization (Rep) as shown in E. The yellow arrows in the snapshots represent the direction of the wavefront propagation with double horizontal lines denoting the site of conduction block. The VT originates from a focal site at the LV base and propagates as single wavefront toward the apex and undergoes functional conduction block at site 3. The two lateral edges of the front, however, continue to propagate laterally (snapshot, 98 ms) forming figure-eight reentry (snapshot, 108 ms). During the second reentrant wavefront, another wavefront emerges from the apical site of the LV (snapshot, 122 ms), disrupting the activation pattern and signaling the onset of VF. D: 3 optical action potentials (APs; labeled 1, 2, and 3) recorded from sites identified on the heart silhouette (C). The 2 downward-pointing blue arrows indicate the direction of propagation from site 1 to site 3 with the red downward-pointing arrow showing block at site 3, followed by retrograde activation (upward-pointing arrow). Notice the emergence of spatially discordant AP duration (APD) alternans preceding conduction block at site 3 when the front with short APD (S) at site 1 encroaches a site (site 3) with long APD (L).
Statistical Analysis
Differences in the Cai2+ decline rate constant and APDs at different pacing cycle lengths (CLs) were determined using repeated-measures two-way ANOVA. Differences among individual means were verified subsequently by Newman-Keuls post hoc tests. Likelihood ratio test was used to determine the significance of site-specific origination of focal activity. P ≤ 0.05 was considered significant. All data are presented as means ± SD.
RESULTS
H2O2 Fails to Induce EAD-Related Arrhythmias in Young Adult Rat and Rabbit Hearts
Isolated Langendorff rat and rabbit hearts were arterially perfused with Tyrode solution and then exposed to H2O2 added directly to the perfusate. All hearts were in regular sinus rhythm after mounting in a tissue bath. In 25 of 25 hearts isolated from young adult rats, the addition of 0.1 mM H2O2 to the perfusate failed to cause spontaneous ventricular arrhythmias during monitoring for up to 3 h. Raising H2O2 concentration up to 2 mM also failed to induce arrhythmias in eight adult rat hearts studied. Similar results were obtained in six young adult (3–5 mo old) rabbit hearts. These findings are consistent with the hypothesis that EADs are suppressed by cell-to-cell coupling in intact tissue, since H2O2 concentrations that consistently induced EADs and TA in isolated rat and rabbit ventricular myocytes (32, 36, 41) failed to induce EAD-related arrhythmias in all adult rat and rabbit intact hearts studied.
H2O2 Promotes Spontaneous VF in the Aged Rat and Middle-Aged Rabbit Hearts
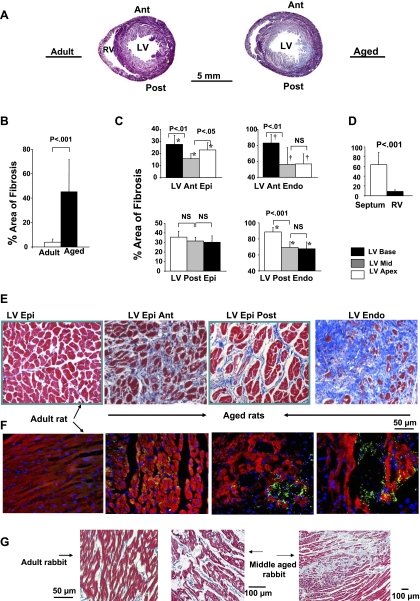
Since aging is associated with increased cardiac fibrosis, which disrupts normal cell-to-cell coupling, we examined whether fibrotic aged hearts are more susceptible to EAD-mediated arrhythmias in response to H2O2. We studied aged rats (24–26 mo) and middle-aged rabbits (3–5 yr) since aged rabbits were not available. Histological analysis using Masson's trichrome collagen staining confirmed earlier report (7) of a marked increase in fibrosis in aged rat ventricles compared with those of adults (Fig. 1A), averaging 45 ± 26% of tissue area (Fig. 1B). The distribution of fibrosis was highly heterogeneous, varying between 10% in the right ventricular (RV) to 90% in the LV endocardium (Fig. 1, C–E). Middle-aged rabbit hearts, however, had less extensive fibrosis. Six of the 10 had 18 ± 5% overall fibrosis, whereas the remaining four middle-aged rabbits had ventricular fibrosis averaging 45 ± 16%, ranging from 15% in the RV and mid-LV wall to 40% in the anterior and posterior LV near the base of the heart (Fig. 1G). In contrast, fibrosis was minimal in adult rat hearts, averaging 4 ± 2.5% of the ventricles and 3 ± 1% in adult rabbit ventricles (P < 0.001 compared with aged rat and middle-aged rabbit ventricles) (Fig. 1, A, B, and G).
Fig. 1.
Masson trichrome and discoidin domain receptor-2 staining in adult and aged ventricles. A: low-power magnification of the entire cross-sectional view of an adult and aged rat hearts. Notice the increased fibrosis in the aged (blue stain) with almost complete fibrosis at the endocardium (Endo) and diffuse fibrosis of the posterior left ventricle (LV) and the septum. B: overall percent area of fibrosis in adult and aged ventricles. C and D: regional variations of percent fibrosis at 14 different ventricular sites. E: trichrome stain that stains collagen blue. F: discoidin domain receptor 2 that stains the fibroblast in green. G: trichrome stain in adult (left) and middle-aged rabbits (middle and right) in which H2O2 induced spontaneous ventricular fibrillation (VF). Ant, anterior; RV, right ventricle; Post, posterior; Epi, epicardium; NS, not significant.
Arrhythmias in aged rat hearts.
In response to 0.1 mM H2O2, 33 of 35 of the aged rat hearts (94%) developed spontaneous VT/VF after a mean perfusion time of 17.6 ± 7.1 min. As shown in Fig. 2A, VF was preceded by a transient period of VT (mean CL of 70 ± 18 ms), which arose suddenly from regular sinus rhythm with a mean CL of 380 ± 162 ms. Within 2 s, the VT degenerated to sustained VF (CL of 55 ± 16 ms) (Fig. 2A), requiring electrical shock for termination. However, VT/VF reoccurred repeatedly within 2 to 3 min after defibrillation in all 33 hearts, allowing us to capture the onset of multiple VT/VF episodes in each heart. Voltage activation maps showed that in each of the 10 mapped hearts, VT had a focal mechanism that preferentially originated from the base of the LV anterior epicardium in 76% of the episodes (68 out of 89 VT episodes in the 10 mapped hearts). A likelihood ratio test indicated that the origination of the focal VT from the LV base in 76% of the VF episodes was not random but a highly significant (P < 0.001) event. The focal VT propagated as single wavefront from the base of the heart to the apex as shown in the snapshots in Fig. 2B, promoting spatially discordant APD alternans (Fig. 2D), causing localized conduction block (wavebreak) midway between the base and the apex of the heart. After the block, the wavefront continued to propagate laterally past the site of the block, forming a figure eight-type reentry (Fig. 2B), coinciding in time with the transition from the VT to VF on the ECG (Fig. 2A). In the remaining 21 episodes, the origin and the mechanism of the transient VT preceding the VF could not be determined. In these episodes, a single wavefront entered the mapped region either from the apical site of the LV (14 out 89 episodes, i.e., 18%) or from the left lateral region of the LV (7 out of 89 episodes, i.e., 6%). The effects of H2O2 were reversible. After washout with H2O2-free Tyrode solution for 20 min, spontaneous VT/VF episodes ceased to reoccur for up to 3 h as observed after H2O2 washout.
Arrhythmias in middle-aged rabbits.
Four of the 10 middle-aged rabbit hearts (40%) with an overall LV fibrosis averaging 45 ± 16% developed similar arrhythmias, culminating in VF after a mean perfusion time of 21 ± 8 min with 0.1 mM H2O2. Figure 3 illustrates an example. As in the case of aged rats, VF was preceded by a transient period of VT (mean CL of 130 ± 20 ms), which arose suddenly from regular sinus rhythm with a mean CL of 460 ± 80 ms. Within 3 s, the VT degenerated to sustained VF (CL of 105 ± 15 ms), requiring electrical shock for termination. However, the reoccurrences of VF after shock-induced termination of VF were less frequent in the middle-aged rabbits compared with aged rats in which repeat spontaneous VF episodes frequently reoccurred. As a result, we could not conduct systematic microelectrode (except 2 episodes of spontaneous VF) and pharmacological studies on VF in the middle-aged rabbits as we did in the aged rats.
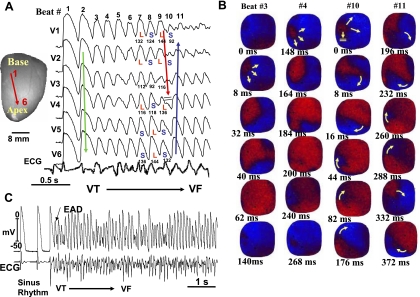
Fig. 3.
Spontaneous initiation of VF in a middle-aged rabbit exposed to 0.1 mM H2O2. A: 6 epicardial optical APs (V1 to V6) recorded from sites shown on the left silhouette of the heart. After 9 focal activations arising from the base of the heart (B), the wavefront undergoes block at mid-LV anterior wall after spatially discordant APD alternans emerges (A). The wavefront, however, continues to propagate lateral to the site of block in a clockwise direction causing a reentrant excitation as shown in the snapshots in B. The numbers under each snapshot is activation time starting with 0 ms (arbitrary) for beats 3 and 4 and then again for beats 10 and 11. C: microelectrode recording of another VF episode recorded in the same heart showing spontaneous initiation of VF by a mechanism compatible with early afterdepolarization (EAD)-mediated triggered activity (TA) as in aged rats shown in Figs. 4 and 5.
Epicardial EAD-Mediated TA Initiates Spontaneous Focal VT
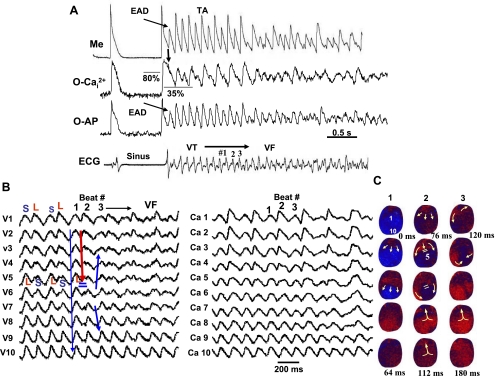
To gain insight into whether EAD-mediated TA was the cause of focal VT, we studied aged rat hearts exposed to H2O2 in greater detail. After terminating VF with an electrical shock, we then continuously recorded with a roving glass microelectrode transmembrane single cell APs from the LV base (the most frequent site of origin of the focal VT) to capture the onset of new VT/VF episodes. With this approach, we successfully captured the onset of 3–5 episodes of VT/VF in each of the 10 aged rat hearts studied. Figure 4 illustrates an example of a VT episode that precedes the VF. As shown in Fig. 4, the VT was initiated by an EAD-mediated TA that arose from a mean takeoff potential of −51 ± 16 mV (42 cells in 10 hearts). The mean CL of the TA was 66 ± 10 ms and was not significantly different from the mean CL of the VT (70 ± 18 ms). The EAD preceded the QRS complex of a simultaneously recorded ECG by a mean of 8 ± 4 ms and occurred during an isoelectric interval on the ECG as shown in Fig. 4A, indicating the absence of electrical activity elsewhere in the heart during the EAD formation. In two middle-aged rabbits, microelectrode recordings at the onset of spontaneous VF (Fig. 3C) showed a similar pattern of VF initiation as in aged rat hearts (Fig. 4) that arose suddenly during the sinus rhythm by a mechanism suggestive of EAD-mediated TA, causing VT and VF. Figure 5A shows that the EADs arise when Cai2+ remains high relative to the diastolic resting level, reflecting a slowed rate of decline of Cai2+ (see Table 1). Interestingly, the rapid TA supported by a single wavefront underwent wavebreak and reentry after the emergence of spatially discordant APD and Cai2+ alternans (Fig. 5, B and C). This dynamic scenario starting with a focal TA causing VT then progressing to VF after the emergence of spatially discordant APD/Cai2+ alternans is compatible with the scenario shown in Figs. 2 and 3.
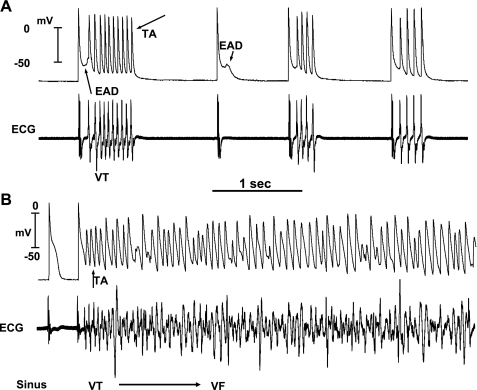
Fig. 4.
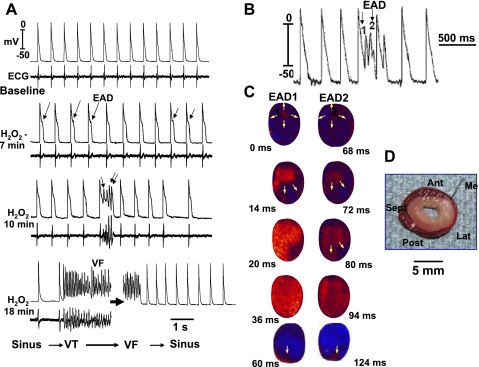
Simultaneous microelectrode and ECG recordings at the onset of VT/VF in an aged rat heart exposed to 0.1 mM H2O2. A: onset of EAD-mediated TA causing VT 5 min after H2O2 exposure. Note the smooth emergence of EAD (upward-pointing arrow) during the isoelectric interval on the ECG followed by a run of 10 TA (downward-pointing arrow) causing nonsustained VT on the ECG. The onset of the EAD precedes the QRS complex of the VT by 8 ms, indicating absence of electrical activity elsewhere in the heart. Two additional short runs of VT with 4 beats each are also shown that follow a single subthreshold EAD (downward small arrow) with no TA. B: degeneration of the TA to VF 15 min after H2O2 exposure.
Fig. 5.
Simultaneous microelectrode (Me) and dual optical AP (O-AP)-intracellular calcium (O-Cai2+) map at the onset of VT/VF in an aged rat heart exposed to H2O2 (0.1 mmol/l). A: single cell APs recorded with glass microelectrode along with simultaneous optical AP-Cai2+ signals recorded within 1 mm of the microelectrode. Notice that the cellular EAD arises when Cai2+ is about 80% of the peak systolic calcium transient amplitude, reflecting a slowed decline rate of Cai2+. The Cai2+ remains above the resting level (35%) during the TA, leading to VF. B: simultaneous 10 voltage and Cai2+ optical signals recorded from base to apex (shown in the snapshots in C) during the transition from VT supported by a single wavefront to wavebreak causing figure-eight reentry (C; beat 1 to beat 2) after the emergence of spatially discordant APD and Cai2+ alternans.
Table 1.
Intracellular calcium decline rate constant (τ) in aged and adult rat hearts
| N | Base | Mid | Apex | P | |
|---|---|---|---|---|---|
| Adult rat | |||||
| Baseline, ms | 8 | 71±5 | 72±5 | 73±5 | NS |
| H2O2, ms | 8 | 80±7 | 82±8 | 83±8 | NS |
| P | <0.05 | <0.05 | <0.05 | ||
| Aged rat | |||||
| Baseline, ms | 14 | 84±6‡ | 76±7‡ | 74±5‡ | <0.05 |
| H2O2, ms | 8 | 110±8‡ | 96±8‡ | 94±10‡ | <0.01 |
| P | <0.01 | <0.01 | <0.01 |
Values are means ± SD; N, number of rats. Repeated-measures ANOVA comparison of intracellular calcium decline rate at different left ventricular (LV) sites during pacing at a cycle length of 200 ms are shown. *P < 0.05, significant difference between adult and aged hearts at all LV sites both at baseline and after H2O2. †P < 0.01, significant regional differences exist in the aged hearts between the base and the mid- and the apex of LV both at baseline and after H2O2. No differences are seen between mid- and apical sites at baseline and after H2O2 in the aged hearts. NS, not significant.
To determine a potential role of epicardial breakthrough activation in the genesis of “focal VT” on the LV epicardial surface, we cryoablated the endocardium and the midmyocardium in five aged rat hearts, sparing only a thin rim of viable epicardium in the LV. As shown in Fig. 6, an exposure of these cryoablated hearts to H2O2 still evoked EADs (total of 13 episodes of EADs in 5 cryoablated hearts with 2 to 3 episodes in each heart) by the surviving epicardial cells at the base of the LV that propagated toward the apex causing VT. These findings indicate that in the LV, the epicardial cells at the base of the rat heart manifest an intrinsic ability to generate EADs, causing focal VT independent of deeper myocardial cells.
Fig. 6.
Cryoablation of mid- and endocardial structures in an aged rat heart and the effect of 0.1 mM H2O2. A: simultaneous microelectrode (top) and ECG (bottom) recordings in a heart subjected to cryoablation of the endo- and midmyocardial structures (whitish area in D). EADs, TA causing VF, are still present after the cryoablation procedure. C: optical snapshots of two EADs (1 and 2 in B) causing focal activation that arises from the base of the surviving thin LV epicardial rim. Snapshots in C show activation time starting with 0 ms (arbitrary) with the second EAD (EAD2) arising 68 ms after the first EAD (EAD1). Only a thin rim of epicardial tissue survives the ablation procedure as indicated by the reddish area in this triphenyltetrazolium chloride-stained cross-sectional view of the LV (D). Me, the site of microelectrode recording shown in A and B; Ant, Sep, Lat, and Post, anterior, septal, lateral, and posterior wall of the LV, respectively. Notice that the VF in this cryoablated heart terminates spontaneously within 1 min of initiation consistent with tissue mass reduction (Ref. 13).
H2O2-Induced Arrhythmias in Aged Rat Hearts Involve Oxidative Activation of CaMKII
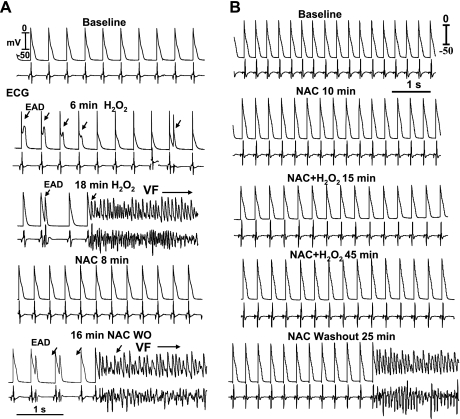
To confirm that H2O2-induced arrhythmias in aged rat hearts, like isolated myocytes, are mediated by oxidative stress, we tested the effects of the reducing agent NAC (2 mM) (21). When the reducing agent NAC was administered, either after H2O2 had already induced VF (N = 5; Fig. 7A) or 15 min before H2O2 (N = 5; Fig. 7B), NAC effectively suppressed and prevented, respectively, the VF in all 10 aged hearts studied. The effect of NAC was reversible. Upon washout of the NAC, the VF reemerged after a mean washout time of 18 ± 5 min (Fig. 6).
Fig. 7.
Suppression and prevention of H2O2-induced EAD and VF in 2 aged rat hearts by N-acetylcysteine (NAC; 2 mM). A and B: simultaneous microelectrode and ECG recordings, showing NAC-induced suppression of EAD-mediated VF and its reemergence 16 min after NAC washout (WO). B: prevention of VF by NAC pretreatment and the emergence of VF 25 min after NAC WO.
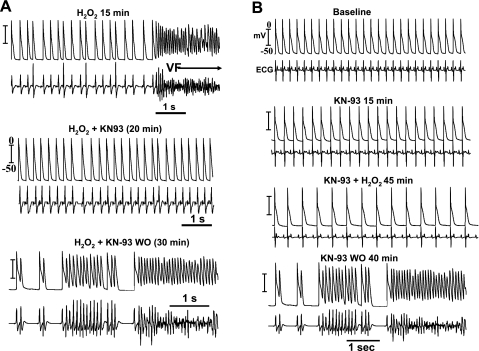
Since H2O2-induced EADs and TA in isolated rabbit myocytes depend on oxidative activation of CaMKII (5) by H2O2 (1, 41), we tested the efficacy of the specific CaMKII inhibitor KN-93 (1 μM) (1, 35) on H2O2-mediated VF in 10 aged rat hearts. When KN-93 was perfused either 15 min after H2O2 had already induced the VF (N = 5; Fig. 8A) or 15 min before H2O2 exposure (N = 5; Fig. 8B), KN-93 suppressed and prevented VF in all 10 hearts, respectively. The effect of KN-93 was reversible. Upon washout of KN-93, the VF reemerged after a mean 14 ± 4 min as shown in Fig. 7. In contrast, KN-92 (1 μM), the inactive form of the KN-93 (1, 35), failed to prevent or suppress H2O2-mediated EADs and VF in all five aged rat hearts tested.
Fig. 8.
Suppression and prevention of H2O2-induced EAD and VF in 2 aged rat hearts by CaMKII inhibitor, KN-93 (1 μM). A: H2O2-mediated VF, which upon 20 min of KN-93 perfusion, the VF is suppressed in the continued presence of H2O2. Upon 30 min of KN-93 WO in the continued presence of H2O2, VF reemerges. B: prevention of VF emergence with KN-93 pretreatment 15 min before the start of H2O2 perfusion. H2O2 exposure for 45 min failed to induced VF; however, upon 40 min of KN-93 WO in the continued presence of H2O2, VF emerges.
These findings suggest that the mechanisms by which H2O2 induces EADs and EAD-mediated arrhythmias in intact aged hearts are the same as in isolated myocytes involving oxidative activation of CaMKII (36).
Two-Dimensional Computer Simulations Support Fibrosis as a Mechanism Promoting EAD-Mediated Arrhythmias
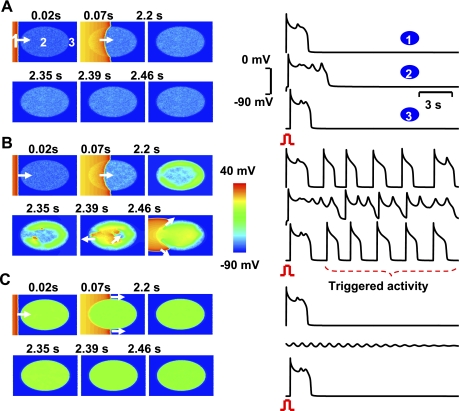
To investigate whether reduced cell-to-cell coupling due to fibrosis is sufficient to account for the emergence of EADs and TA in aged hearts exposed to oxidative stress, we performed computer simulations in 2-D cardiac tissue in which the ratio of fibroblasts to ventricular myocytes coupling was varied (supplemental Fig. 1) using a rabbit ventricular AP model (18). Longitudinal coupling between myocytes had a gap junction conductance of 600 nS, and H2O2-mediated EADs were simulated by doubling the maximum conductance of INCX (6, 17) and peak ICa-L (39, 41), decreasing the Cai2+ transient decline rate by 20% (12) and by maintaining 0.04 of the peak INa during the repolarization phase to mimic the late INa (32, 35, 36). As shown in Fig. 9, when the fibroblast-to-myocyte ratio was in the range of 0.5 to 1 (equivalent to 10–20% by area); EADs that arose after a paced beat were of relatively low amplitude and failed to propagate (Fig. 9A). Similarly, when the fibroblast-to-myocyte ratio was increased to 3:1 (60% by area), the EADs were also of low amplitude and failed to propagate (Fig. 9C). However, when the fibroblast-to-myocyte ratio was in an intermediate range of 1.3–1.5 (25–30% by area), EADs were of sufficient amplitude to propagate into the surrounding tissue, causing repetitive TA (Fig. 9B).
Fig. 9.
Two-dimensional simulations in a tissue with progressive regional increase in the number of fibroblasts to myocyte gap junction couplings. A–C: voltage snapshots after a paced beat from the left border of the tissue (red) with central ellipse (site 2) representing the region of progressive increase in fibroblast to myocyte-coupling ratio (F/M). In A, only small amplitude nonpropagating EADs occur when F/M equals 1. Similar dynamic scenario is also observed when the F/M in the ellipse is raised to 3 as shown in C. After the pace beat, EADs die out locally. However, when the F/M is in the intermediate range (1.1; B), the EADs are now of higher amplitude that propagate and excite the adjacent cardiac tissue as triggered beats. (see supplemental schematic diagram).
These findings agreed well with the histological analysis in aged rat hearts when we compared the percent tissue fibrosis at the site of frequent origination of EADs and TA (i.e., the LV epicardial base) to 13 other LV and RV sites. As shown in Fig. 1C, the site of frequent EAD origination had an intermediate percent fibrosis (mean of 28%) compared with RV (4%) and to mid- and apical LV anterior epicardium (10–20%) and posterior LV epicardial, endocardial, and septal regions (40–90%) (Fig. 1, D and E). Fibrosis was characterized both by increased collagen deposits (Fig. 1, E and G) and increased fibroblast density as shown by discoidin domain receptor 2 staining of fibroblast receptors (3) (Fig. 1F).
Electrophysiological Differences Between Young Adult and Aged Rat Hearts
Although the computer simulations (Fig. 9) support increased fibrosis in aged hearts as a plausible explanation for their increased susceptibility to H2O2-induced arrhythmias, aging-related changes in electrophysiological and calcium cycling properties at the myocyte level may also be important. In comparing young adult versus aged rat hearts, however, we found that H2O2 had no significant effect on APD90, APD50, or the maximum APD restitution slope, both before and after exposure to H2O2 (see supplemental Table). On the other hand, some differences in Cai2+ cycling between young adult and aged rat hearts were noted. The mean rate constant τ of the Cai2+ transient decline (fit to a single exponential) was significantly longer in aged compared with young adult ventricles (P < 0.05), both before and after H2O2 exposure (Table 1). Furthermore, there was greater regional heterogeneity in Cai2+ handling in the aged than in the young adult rat hearts, with the slowest τ clustering at the base of the LV compared with mid- and apical sites (Table 1).
DISCUSSION
The major finding of this study is that when aged hearts are exposed to a level of H2O2-induced oxidative stress that is benign in young adult hearts, they are highly susceptible EAD-mediated arrhythmias and VF. The high reproducibility of spontaneous VT/VF in aged rat hearts makes this model potentially valuable for studying VF episodes arising abruptly during otherwise stable sinus rhythm without prior heart rate acceleration and/or visible T-wave alternans of the sinus beats (19). This study also highlights the influence of cell-to-cell coupling on the ability of EADs to form and cause arrhythmias in tissue. Isolated young adult rat and rabbit ventricular myocytes readily develop EADs and TA in response to 0.1 mM H2O2.(32, 36, 41). However, young adult rat and rabbit hearts exhibited no arrhythmias under the same conditions. This discrepancy supports the supposition that cell-to-cell coupling is a potent mechanism suppressing EAD formation in tissue by creating a source-sink mismatch that prevents local EAD currents generated by a small group of myocytes from reversing repolarization when they are electrotonically coupled to a large group of adjacent normally repolarizing myocytes.
To explore the effects of cell-to-cell coupling on EAD suppression, we performed computer simulations of 2-D cardiac tissue in which normal myocyte-to-myocyte coupling was disrupted by inserting fibroblasts into the tissue. With too few fibroblasts, EADs and TA were suppressed by cell-to-cell coupling, and with too many fibroblasts, EADs occurred but were unable to propagate. With an intermediate myocyte-to-fibroblast ratio, however, EADs both formed and successfully propagated into the surrounding tissue. These findings agreed well with the experimental results in aged rat hearts in which EADs and TA typically arose from regions with intermediate levels of fibrosis (30–40%), usually at the base of the LV epicardium. Importantly, our experiments in cyroablated aged rat hearts demonstrated that EADs were generated in the LV epicardium itself, rather than arising from the underlying tissue or the His-Purkinje system.
Although the disruption of normal cell-to-cell coupling by fibrosis provides a plausible explanation for the increased susceptibility of aged hearts to EADs and EAD-mediated arrhythmias, we cannot exclude the possibility that other aspects of aging-related remodeling (e.g., electrical, Cai2+ cycling, and gap junction remodeling) also make important contributions. Nevertheless, isolated myocytes from young adult rat (or rabbit) ventricular myocytes developed frank EADs when exposed to H2O2 (32, 36, 41), even though intact young adult hearts never developed EADs, TA, or VT/VF when perfused with the same or even 20-fold higher H2O2 concentrations for up to 3 h. These findings indicate that young adult myocytes are clearly susceptible to H2O2-induced EADs, but something in the tissue environment suppresses their emergence. Our findings support a critical role of partial cellular uncoupling in EAD formation at the tissue level caused by increased interstitial tissue fibrosis.
The mechanisms by which H2O2 induced EADs in isolated young adult ventricular myocytes and aged hearts seem to be identical, since both were suppressed by CaMKII inhibitors and antioxidants (41), consistent with the oxidative activation of CaMKII as the underlying molecular mechanism (40). It might be speculated that intact young adult hearts are less susceptible to H2O2-induced EADs because they have greater antioxidant capacity than aged hearts (8). However, this is unlikely since increasing perfusate H2O2 concentration 20-fold (2 mM H2O2) still failed to induce EADs, TA, VT, and VF in young adult rat hearts. Young adult and aged rat hearts also exhibited no differences in APD or APD restitution during regular pacing at different pacing CLs, either before or after oxidative stress. On the other hand, the rate of Cai2+ transient decline was significantly slower in aged than young adult ventricles, especially after H2O2, which directly inhibits sarco(endo)plasmic reticulum calcium-ATPase 2a (28). We cannot exclude that this effect promoted EADs by elevating Cai2+ during repolarization (23, 34). It may also have enhanced the susceptibility of aged hearts to Cai2+ and APD alternans (30), since depressed sarcoplasmic reticulum Cai2+ uptake is known to promote Cai2+ alternans (25, 37). Once APD alternans has developed, fibrosis is known to promote its transition to spatially discordant APD alternans (26). The combined effects of these two factors is likely to account for the experimental observation that during focal VT, spatially discordant APD alternans preceded the degeneration of VT to VF.
Potential Clinical Impact
The clinical implications of our findings can be summarized as follows: VF is the most common cause of sudden cardiac death and prematurely claims the lives of ∼300,000 persons every year in the United States (29). Many known triggers of oxidative stress, such as aging (11), heart failure (10), and ischemia-reperfusion (2), are linked to an increased risk of VF. Diffuse tissue fibrosis is also a common feature of diverse heart abnormalities, including heart failure (24), myocardial ischemia/infarction, and cardiomyopathy (38). Decreasing oxidative stress and preventing and/or reversing tissue fibrosis in these cardiac conditions may therefore be a promising novel strategy to reduce VF risk and possibly AF risk as well (4). The aged heart model could contribute to the understanding of VF triggers in patients that do not manifest visible T-wave alternans and/or heart rate acceleration before the onset of the VF episode.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants P01-HL-78931, R01-HL-78932, R01-HL-58533, R01-HL-66389, and R01-HL-71140; American Heart Association Western Affiliate Fellowship Award 0725218Y and Grant-in Aid 0555057Y; Laubisch, Kawata, Medtronic-Zipes Endowments; and Electrocardiographic Heart Beat Organization (Cedars-Sinai Medical Center, Los Angeles, CA).
DISCLOSURES
H. S. Karagueuzian has a research award from Gilead Sciences, Inc., Palo Alto, CA.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jong Hwan Lee, John Parker, Shuzen Zhang, Nicole Petrochuk, Hong-Mei Li, and Longshen Hong for valuable technical assistance and ATS Medical (Minneapolis, MN) for donating a cryoablation unit as an unrestricted gift.
REFERENCES
- 1.Anderson ME, Braun AP, Wu Y, Lu T, Wu Y, Schulman H, Sung RJ. KN-93, an inhibitor of multifunctional Ca++/calmodulin-dependent protein kinase, decreases early afterdepolarizations in rabbit heart. J Pharmacol Exp Ther 287: 996–1006, 1998 [PubMed] [Google Scholar]
- 2.Bolli R, Patel BS, Jeroudi MO, Lei EK, McCay PB. Demonstration of free radical generation in “stunned” myocardium of intact dogs with the use of the spin trap alpha-phenyl N-tert-butyl nitrone. J Clin Invest 82: 476–485, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camelliti P, Devlin GP, Matthews KG, Kohl P, Green CR. Spatially and temporally distinct expression of fibroblast connexins after sheep ventricular infarction. Cardiovasc Res 62: 415–425, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Dudley SC, Jr, Hoch NE, McCann LA, Honeycutt C, Diamandopoulos L, Fukai T, Harrison DG, Dikalov SI, Langberg J. Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: role of the NADPH and xanthine oxidases. Circulation 112: 1266–1273, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 133: 462–474, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldhaber JI. Free radicals enhance Na+/Ca2+ exchange in ventricular myocytes. Am J Physiol Heart Circ Physiol 271: H823–H833, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Hacker TA, McKiernan SH, Douglas PS, Wanagat J, Aiken JM. Age-related changes in cardiac structure and function in Fischer 344 × Brown Norway hybrid rats. Am J Physiol Heart Circ Physiol 290: H304–H311, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Hagen TM. Oxidative stress, redox imbalance, and the aging process. Antioxid Redox Signal 5: 503–506, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Hwang GS, Tang L, Joung B, Morita N, Hayashi H, Karagueuzian HS, Weiss JN, Lin SF, Chen PS. Superiority of biphasic over monophasic defibrillation shocks is attributable to less intracellular calcium transient heterogeneity. J Am Coll Cardiol 52: 828–835, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ide T, Tsutsui H, Kinugawa S, Suematsu N, Hayashidani S, Ichikawa K, Utsumi H, Machida Y, Egashira K, Takeshita A. Direct evidence for increased hydroxyl radicals originating from superoxide in the failing myocardium. Circ Res 86: 152–157, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Jahangir A, Sagar S, Terzic A. Aging and cardioprotection. J Appl Physiol 103: 2120–2128, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Kennedy DJ, Vetteth S, Xie M, Periyasamy SM, Xie Z, Han C, Basrur V, Mutgi K, Fedorov V, Malhotra D, Shapiro JI. Ouabain decreases sarco(endo)plasmic reticulum calcium ATPase activity in rat hearts by a process involving protein oxidation. Am J Physiol Heart Circ Physiol 291: H3003–H3011, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Kim YH, Garfinkel A, Ikeda T, Wu TJ, Athill CA, Weiss JN, Karagueuzian HS, Chen PS. Spatiotemporal complexity of ventricular fibrillation revealed by tissue mass reduction in isolated swine right ventricle. Further evidence for the quasiperiodic route to chaos hypothesis. J Clin Invest 100: 2486–2500, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohl P. Heterogeneous cell coupling in the heart: an electrophysiological role for fibroblasts. Circ Res 93: 381–383, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Kohl P, Kamkin AG, Kiseleva IS, Noble D. Mechanosensitive fibroblasts in the sino-atrial node region of rat heart: interaction with cardiomyocytes and possible role. Exp Physiol 79: 943–956, 1994 [DOI] [PubMed] [Google Scholar]
- 16.MacCannell KA, Bazzazi H, Chilton L, Shibukawa Y, Clark RB, Giles WR. A mathematical model of electrotonic interactions between ventricular myocytes and fibroblasts. Biophys J 92: 4121–4132, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mace LC, Palmer BM, Brown DA, Jew KN, Lynch JM, Glunt JM, Parsons TA, Cheung JY, Moore RL. Influence of age and run training on cardiac Na+/Ca2+ exchange. J Appl Physiol 95: 1994–2003, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Mahajan A, Sato D, Shiferaw Y, Baher A, Xie LH, Peralta R, Olcese R, Garfinkel A, Qu Z, Weiss JN. Modifying L-type calcium current kinetics: consequences for cardiac excitation and arrhythmia dynamics. Biophys J 94: 411–423, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller MA, Gomes JA, Fuster V. Risk stratification of sudden cardiac death in hypertrophic cardiomyopathy. Nat Clin Pract Cardiovasc Med 4: 667–676, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Miyauchi M, Qu Z, Miyauchi Y, Zhou SM, Pak H, Mandel WJ, Fishbein MC, Chen PS, Karagueuzian HS. Chronic nicotine in hearts with healed ventricular myocardial infarction promotes atrial flutter that resembles typical human atrial flutter. Am J Physiol Heart Circ Physiol 288: H2878–H2886, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Murphy AJ. Sulfhydryl group modification of sarcoplasmic reticulum membranes. Biochemistry 15: 4492–4496, 1976 [DOI] [PubMed] [Google Scholar]
- 22.Ono N, Hayashi H, Kawase A, Lin SF, Li H, Weiss JN, Chen PS, Karagueuzian H. Spontaneous atrial fibrillation initiated by triggered activity near the pulmonary veins in aged rats subjected to glycolytic inhibition. Am J Physiol Heart Circ Physiol 292: H639–H648, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Patterson E, Lazzara R, Szabo B, Liu H, Tang D, Li YH, Scherlag BJ, Po SS. Sodium-calcium exchange initiated by the Ca2+ transient: an arrhythmia trigger within pulmonary veins. J Am Coll Cardiol 47: 1196–1206, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res 88: 1159–1167, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Pruvot EJ, Katra RP, Rosenbaum DS, Laurita KR. Role of calcium cycling versus restitution in the mechanism of repolarization alternans. Circ Res 94: 1083–1090, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Qu Z, Karagueuzian HS, Garfinkel A, Weiss JN. Effects of Na+ channel and cell coupling abnormalities on vulnerability to reentry: a simulation study. Am J Physiol Heart Circ Physiol 286: H1310–H1321, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Rook MB, van Ginneken AC, de Jonge B, el Aoumari A, Gros D, Jongsma HJ. Differences in gap junction channels between cardiac myocytes, fibroblasts, and heterologous pairs. Am J Physiol Cell Physiol 263: C959–C977, 1992 [DOI] [PubMed] [Google Scholar]
- 28.Rowe GT, Manson NH, Caplan M, Hess ML. Hydrogen peroxide and hydroxyl radical mediation of activated leukocyte depression of cardiac sarcoplasmic reticulum. Participation of the cyclooxygenase pathway. Circ Res 53: 584–591, 1983 [DOI] [PubMed] [Google Scholar]
- 29.Rubart M, Zipes DP. Mechanisms of sudden cardiac death. J Clin Invest 115: 2305–2315, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato D, Shiferaw Y, Qu Z, Garfinkel A, Weiss JN, Karma A. Inferring the cellular origin of voltage and calcium alternans from the spatial scales of phase reversal during discordant alternans. Biophys J 92: L33–L35, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato D, Xie LH, Sovari AA, Tran DX, Morita N, Xie F, Karagueuzian H, Garfinkel A, Weiss JN, Qu Z. Synchronization of chaotic early afterdepolarizations in the genesis of cardiac arrhythmias. Proc Natl Acad Sci USA 106: 2983–2988, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song Y, Shryock JC, Wagner S, Maier LS, Belardinelli L. Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther 318: 214–222, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Vasquez C, Moreno AP, Berbari EJ. Modeling fibroblast-mediated conduction in the ventricle. Comput Cardiol 31: 349–352, 2004 [Google Scholar]
- 34.Volders PG, Vos MA, Szabo B, Sipido KR, de Groot SH, Gorgels AP, Wellens HJ, Lazzara R. Progress in the understanding of cardiac early afterdepolarizations and torsades de pointes: time to revise current concepts. Cardiovasc Res 46: 376–392, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, Maier SK, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest 116: 3127–3138, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ward CA, Giles WR. Ionic mechanism of the effects of hydrogen peroxide in rat ventricular myocytes. J Physiol 500: 631–642, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss JN, Karma A, Shiferaw Y, Chen PS, Garfinkel A, Qu Z. From pulsus to pulseless: the saga of cardiac alternans. Circ Res 98: 1244–1253, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Wu TJ, Ong JJ, Hwang C, Lee JJ, Fishbein MC, Czer L, Trento A, Blanche C, Kass RM, Mandel WJ, Karagueuzian HS, Chen PS. Characteristics of wave fronts during ventricular fibrillation in human hearts with dilated cardiomyopathy: role of increased fibrosis in the generation of reentry. J Am Coll Cardiol 32: 187–196, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Wu Y, MacMillan LB, McNeill RB, Colbran RJ, Anderson ME. CaM kinase augments cardiac L-type Ca2+ current: a cellular mechanism for long Q-T arrhythmias. Am J Physiol Heart Circ Physiol 276: H2168–H2178, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Wu Y, Temple J, Zhang R, Dzhura I, Zhang W, Trimble R, Roden DM, Passier R, Olson EN, Colbran RJ, Anderson ME. Calmodulin kinase II and arrhythmias in a mouse model of cardiac hypertrophy. Circulation 106: 1288–1293, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Xie LH, Chen F, Karagueuzian HS, Weiss JN. Oxidative stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ Res 104: 79–86, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.