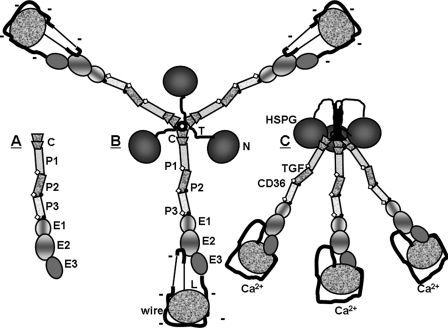
FIGURE 1.
Schematics of (A) TSP-1 stalk modules studied in this paper, (B) TSP-1 in its Ca2+-depleted conformation, and (C) TSP-1 in its Ca2+-replete formation. Parts of TSP-1 in panels A and B are labeled as follows: N, N-terminal module; T, tether; C, vWF-C module; P, properdin or TSR module, E, EGF-like module; wire, Ca2+-binding repeats with 26 Ca2+-binding sites; and L, lectin-like module. The TSP-type EGF-like modules, E1 and E2, contain central shading. Sites of binding to heparin sulfate proteoglycan (HSPG), latent transforming growth factor-β (TGF), and CD36 are indicated in panel C. The schematics have been drawn based on structures described in the text. Sites of fucosylation of TSRs are indicated by open diamonds, and inter-module CPIXG sequences between P2 and P3 and between P3 and E1 are indicated with dots. As per the “Discussion,” changes in conformation and charge density of the signature domain due to gain or loss of Ca2+ are proposed to be propagated throughout trimeric TSP-1 by the stalk modules.