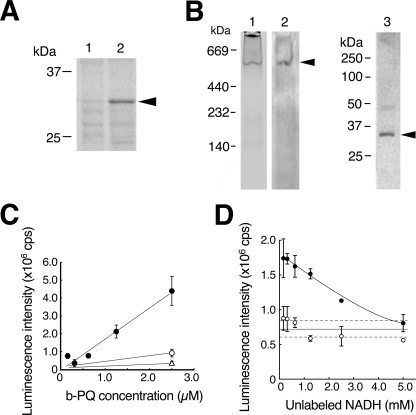
FIGURE 4.
VDAC1 is a component of NADH-PQ oxidoreductase. A, extracts obtained from the mitochondrial outer membrane by treatment with Triton X-100/deoxycholate (lane 1) or SDS/ Igepal CA-630 (lane 2) were run on SDS-PAGE, and the results were analyzed by Western blotting with anti-VDAC1 mAb. VDAC1 protein was detected by the mAb (arrowhead). B, DEAE fractions from the extracts containing oxidoreductase activity were examined by zymography with NADH and PQ in blue tetrazolium solution (lane 1). The active band was consistent with the anti-VDAC1 mAb-detected band (lane 2, arrowhead), and this band was excised and subjected to Western blot analysis using anti-VDAC1 mAb (lane 3; the arrowhead indicates VDAC1 protein). C, direct binding to VDAC1 was assayed using biotinylated (b-) PQ (closed circle) in competition with non-labeled PQ (open circle, 25 μm, open triangle, 250 μm). Error bars represent S.D. (n = 3). D, assay of NADH binding to the outer membrane extracts was performed. The extracts were trapped by immobilized anti-VDAC1 antibody and incubated with biotinylated NAD+. Bound biotinylated NAD+ was reduced by exposure to non-labeled NADH (p < 0.01, closed circles). When normal IgG was used for trapping, no NADH competition was detected (open circles). Broken lines represent 95% confidence interval of the control value. Error bars represent S.D. (n = 3).