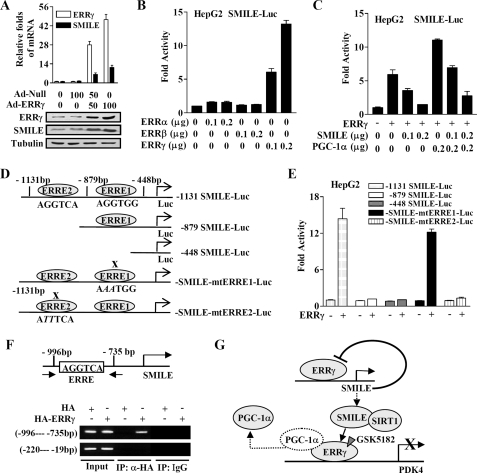
FIGURE 7.
Autoregulatory loop controlling SMILE gene expression by ERRγ. A, upper panel shows the relative ERRγ and SMILE mRNA levels analyzed by quantitative real time PCR (standardized using β-actin). Normalized basal levels of each transcript were assigned an arbitrary value of 1.0 for comparison. Lower panel shows the protein expression levels of ERRγ and SMILE. Tubulin was used as a loading control. HepG2 cells were infected with adenovirus vector (Ad-Null and Ad-ERRγ) at indicated multiplicity of infection (0, 50, or 100). B, ERRγ activates human SMILE promoter activity. HepG2 cell were cotransfected with 0.1 μg of SMILE-Luc reporter vector and indicated amount of expression vectors encoding ERRα, ERRβ, or ERRγ. C, SMILE inhibits ERRγ-mediated and PGC-1α-enhanced SMILE promoter activity. HepG2 cells were cotransfected with 0.2 μg of SMILE-Luc reporter together with or without 0.2 μg of ERRγ expression vector and indicated amount of expression plasmids for SMILE and PGC-1α. D, schematic representation of wild-type and mutant hSMILE promoter constructs. The putative ERRγ binding sites are shown, and the mutated ERRE is indicated with X. E, ERRE2 is essential for the activation of SMILE promoter by ERRγ. HepG2 cells were cotransfected with 0.2 μg of wild-type or mutant SMILE promoter constructs along with or without 0.2 μg of ERRγ expression vector. Luciferase activity was measured 48 h after transfection. F, ERRγ binds to SMILE promoter in ChIP assays. HepG2 cells were transfected with expression vector for HA or HA-ERRγ. Chromatin fragments were prepared from the transfected cells and immunoprecipitated with anti-HA antibody or unrelated immunoglobin G (IgG) as indicated. DNA fragments covering ERRE (−996 to −735 bp) on SMILE promoter and a control region (−220 to −19 bp) were PCR-amplified. Data shown are representative of three experiments. G, schematic representation of the autoregulatory loop controlling the expression of SMILE by ERRγ and the mechanisms of SMILE repression on ERRγ, recruitment of SIRT1 and dissociation of coactivator PGC-1α.