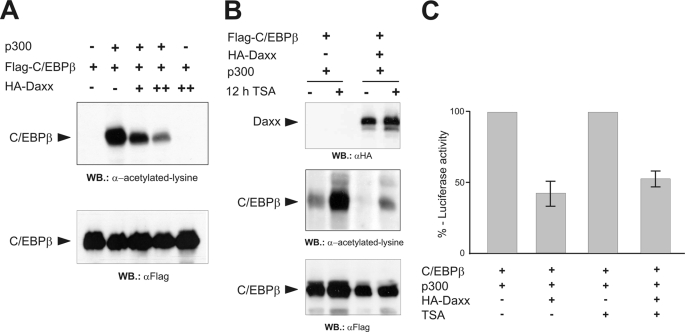
FIGURE 5.
Daxx inhibits the p300-mediated acetylation of C/EBPβ. A, QT6 cells were transfected with pCMVβ (0.5 μg) and expression constructs for FLAG-C/EBPβ (5 μg), HA-Daxx (5 and 10 μg), and HA-p300 (10 μg), as indicated. 24 h later protein extracts were prepared, and β-galactosidase-normalized protein amounts were analyzed by Western blotting using antibodies against acetyllysine or the FLAG tag. B, QT6 cells were transfected with pCMVβ and expression vectors for FLAG-C/EBPβ (1 μg), Ha-Daxx (5 μg), and full-length p300 (5 μg), as indicated at the top. Cells were treated with 400 nm TSA for 12 h before harvesting (+) or were left untreated (−). β-Galactosidase-normalized protein amounts were analyzed by Western blotting using antibodies against Daxx, acetyllysine, or the FLAG tag. Because smaller amounts of C/EBPβ expression vector were transfected in the experiment shown in B as compared with A, the signal intensities in the second lane of A and the first lane of B are different. C, QT6 cells were transfected with the indicated combinations of expression vectors for FLAG-C/EBPβ (1 μg), HA-p300 (5 μg), and HA-Daxx (5 μg) together with the C/EBPβ-responsive p240-Luc reporter construct (3 μg) and pCMVβ (0.5 μg). Cells were treated additionally with TSA as described in panel B or left untreated. Cells were harvested after 24 h, and luciferase and β-galactosidase activities were determined. The columns show the average luciferase activity normalized to the β-galactosidase activity. The activity of the reporter gene in the absence of Daxx was set to 100% in each case.