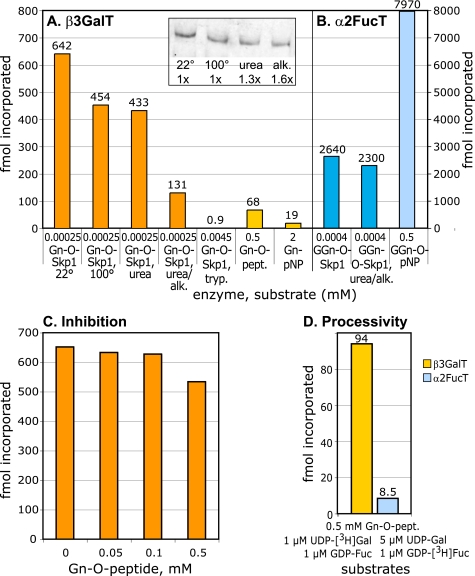
FIGURE 7.
Acceptor substrate specificity of PgtA. A, β3GalT activity. Purified PgtA was incubated with the indicated acceptor in the presence of 1 μm UDP-[3H]Gal for 2 h at 29 °C. Gn-O-Skp1 was isolated from pgtA− cells, and pretreated as indicated. Gn-O-Skp1 was repurified after treatment with 8 m urea or urea and iodoacetamide (alk.), and the volume assayed was corrected by a factor based on recovery as determined by Western blotting of identical volumes (inset). Assays using Skp1 were quantitated by the SDS-PAGE method and background values (<40 dpm) were subtracted. Trypsinized Skp1, which was not detectable by Western blotting (data not shown), was treated with soybean trypsin inhibitor, aprotinin and phenylmethylsulfonyl fluoride, which do not inhibit PgtA. Incorporation into glycopeptides was assayed using the HPLC method, and incorporation into GlcNAcα1-pNP was assayed using the C18-SepPak method. B, α2FucT activity. Purified PgtA was assayed similarly in the presence of 1 μm GDP-[3H]Fuc. GGn-O-Skp1 was isolated from strain HL250, and pretreated as indicated. C, sensitivity of the GalT activity to the Skp1 glycopeptide. The GalT reaction using Gn-O-Skp1 described in panel A was conducted in the presence of the indicated concentration of synthetic glycopeptide. D, reaction using Gn-O-peptide was tested for processivity by conducting the reaction in the presence of the indicated concentrations of UDP-Gal and GDP-Fuc. The GalT and FucT reactions were monitored in parallel reactions using UDP-[3H]Gal and GDP-[3H]Fuc, respectively. Incorporation of [3H]Fuc in absence of UDP-Gal was subtracted as background. The FucT reaction was much slower despite the 5× higher level of UDP-Gal. 1 fmol is 44 dpm in these reactions. All results were confirmed in independent trials.