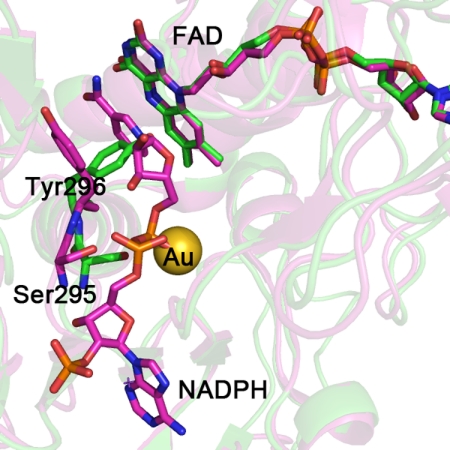
FIGURE 2.
The SmTGR crystal structure in complex with gold ions (in green) is superimposed to the mouse TR with NADPH bound (in magenta; Protein Data Bank code 1zdl (30)). The root mean square deviation is 0.82 Å over the 462 aligned residues. The residues surrounding NADPH in mouse TR are conserved in SmTGR (sequence alignment not shown). The structural comparison shows the change in conformation of the loop 293–296 and in particular of Tyr296 and Ser295, highlighted for the two enzymes as balls and sticks (the other amino acid side chains are omitted for clarity). The OG atom of Ser295 is the closest contact with the gold ion in the SmTGR crystal structure (see “Results” and Fig. 1). In the mouse TR structure, Ser295 shifts in position to make room for the bound NADPH; the clash between the metal in site 3 and the phosphate of the cofactor in SmTGR is self-evident.