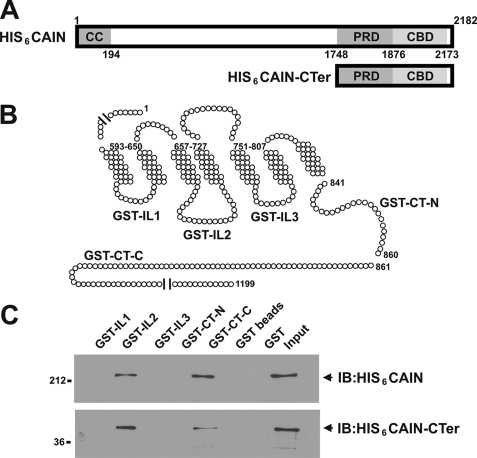
FIGURE 3.
Purified His6-CAIN binds to GST fusion proteins corresponding to the mGluR1a second intracellular loop and distal C-terminal tail domains. A, shown are the full-length CAIN and CAIN C-terminal domains purified from E. coli as His6 fusion proteins and the localization of the coil-coiled (CC) domain, the proline-rich domain (PRD) that binds amphiphysin, and the calcineurin-binding domain (CBD). B, the schematic representation of mGluR1a illustrates the regions of mGluR1a that were prepared as GST fusion proteins. The GST fusion proteins correspond to the first (GST-IL1), second (GST-IL2), and third (GST-IL3) intracellular loops as well as the proximal (GST-CT-N) and distal (GST-CT-C) C-terminal tails. C, 1 μg of purified GST-IL1, GST-IL2, GST-IL3, GST-CT-N, and GST-CT-C was incubated with 1 μg of either full-length His6-tagged CAIN (HIS6CAIN) or the His6-tagged CAIN C-terminal domain (HIS6CAIN-CTer). CAIN eluted with the GST fusion proteins was determined by immunoblotting (IB) with rabbit anti-CAIN polyclonal antibody. Data are representative of three independent experiments.