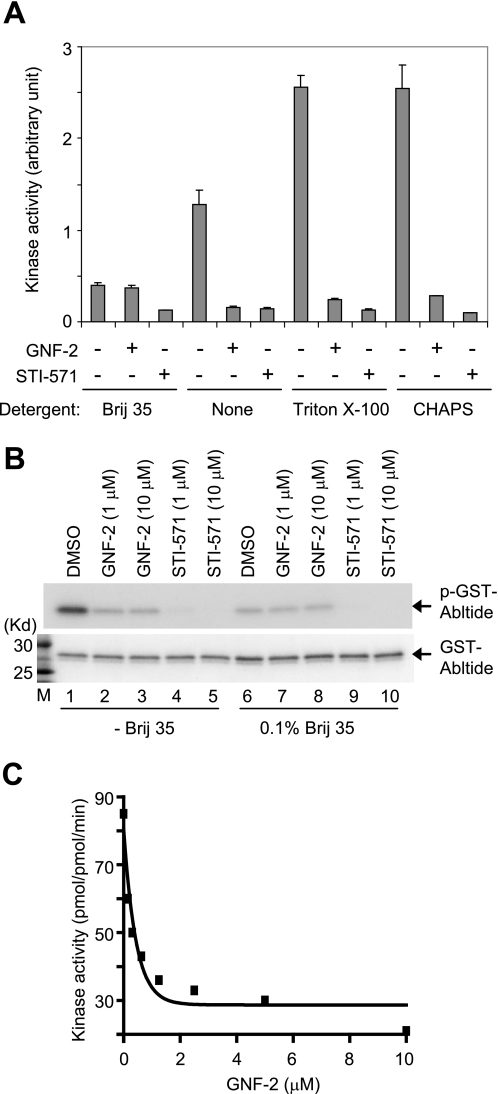
FIGURE 2.
GNF-2 inhibits recombinant Abl kinase activity in vitro. A, recombinant Abl (amino acids 65–534) was diluted in kinase buffer containing 0.1% of the indicated detergents, incubated with either DMSO or 10 μm compounds for 30 min, and then its tyrosine kinase activity was measured by an ELISA-based assay. It is of note that Brij 35 interferes with recombinant Abl kinase activity as well as the ability of GNF-2 to inhibit recombinant Abl kinase activity. B, recombinant Abl (amino acids 65–534) was diluted in kinase assay buffer in the absence or presence of 0.1% Brij 35, incubated with either DMSO or compounds for 30 min, and then its tyrosine kinase activity was assessed by radioenzymatic assay. The phosphorylation of GST-Abltide was monitored by SDS-PAGE and autoradiography (top). The total substrate used (GST-Abltide) was visualized by Coomassie staining (bottom). M, molecular weight marker. C, a continuous spectrophotometric assay confirms the GNF-2 inhibition of recombinant Abl kinase activity. The recombinant Abl (amino acids 65–534) was diluted to a final concentration of 50 nm in kinase buffer (50 mm Tris-Cl (pH 7.4), 50 mm KCl, 10 mm MgCl2) in the absence or presence of GNF-2. Kinase activity was measured with 300 μm substrate and 500 μm ATP for 20 min and expressed as pmol/pmol·min.