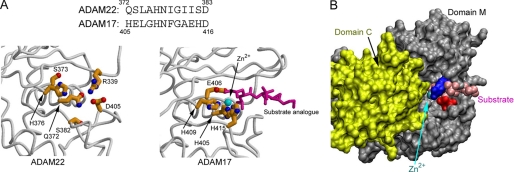
FIGURE 2.
Structural features ensuring domain M to be catalytically inactive. A, a comparison between the ADAM17 catalytic site (PDB ID 1BKC) and the equivalent position in ADAM22 showing that zinc binding and substrate cleavage are disabled in ADAM22 due to the replacement of critical residues. The zinc ion is depicted as a cyan ball, and the substrate analogue is depicted as magenta sticks in ADAM17. B, modeling the zinc ion and the substrate analogue of the ADAM17 structure into ADAM22 based on domain M superimposition showing that substrate binding is disabled in ADAM22 by the salt bridge between Arg-339 (blue) and Asp-405 (red), the main chain of the Arg-339-harboring loop, and the cysteine-rich domain nearby (yellow).