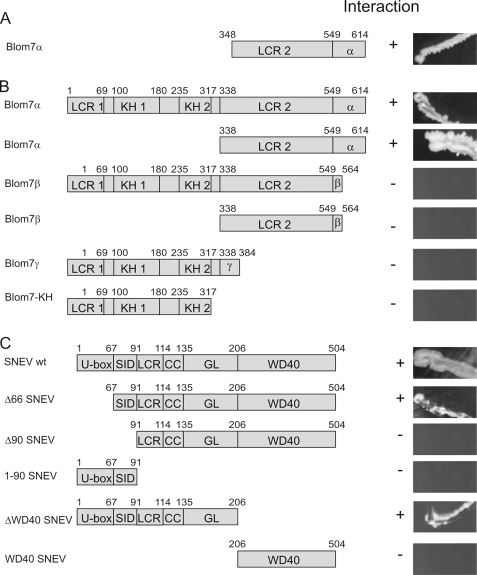
FIGURE 2.
Blom7α-specific C terminus and self-interaction domain of SNEVPrp19-Pso4 are necessary for interaction. A, C-terminal half of amino acid 347–614 were isolated from the human aorta cDNA library. B, Blom7α deletion mutants identify the α-specific C terminus as necessary for the interaction, whereas on the side of SNEVPrp19-Pso4. C, the self-interaction domain is necessary as determined by directed yeast two-hybrid analyses under high stringent conditions. U-box, UFD2-like motif with E3 ligase activity. SID, self-interaction domain; LCR, low complexity region; CC, coiled-coil domain, GL2, globular domain; LCR1, low complexity region APG-rich; KH1, -2, KH domain (RNA binding domain); LCR2, low complexity region PS-rich; α, α-specific C terminus; β, β-specific C terminus; γ, γ-specific C terminus.