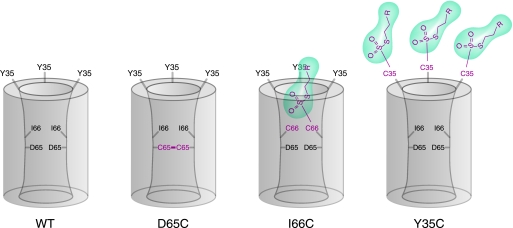
FIGURE 11.
Model of claudin-2 showing the putative location of the mutated residues in the first extracellular domain. The pore is hypothesized to be a homomultimer (residues from 2–3 subunits are shown). Wild-type claudin-2 (WT) is depicted in the left panel, and the consequences of cysteine mutagenesis are shown in the other panels. D65 is located in the narrowest part of the pore facing the lumen and close to an intersubunit interface, so that the D65C mutation leads to dimerization by disulfide bonding. Ile66 is also within the pore facing the lumen, but residues from neighboring subunits are further apart, so that disulfide bonding in I66C is precluded. Thiol-reactive MTS reagents (green) enter the pore to react with I66C, partially blocking the pore to ion permeation. Only a single MTS molecule is accommodated in each pore. Tyr35 is outside the pore facing extracellularly. Thus, reaction of MTS reagents with Y35C does not block the pore. Furthermore, because there is no steric restriction, every Y35C residue can react with an MTS molecule, so that multiple MTS molecules are associated with each pore multimer.