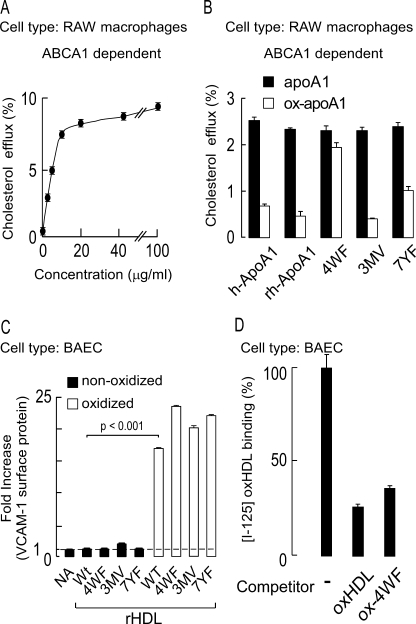
FIGURE 8.
ApoA1 tyrosine, tryptophan, and methionine residues do not appear to be involved in endothelial activation by MPO-oxidized HDL. A, dose-response curve of isolated human apoA1-mediated cholesterol efflux activity (ABCA1-dependent) from cholesterol-laden RAW macrophages. B, ABCA1-mediated cholesterol efflux activity of various apoA1 in the absence versus presence of MPO-catalyzed oxidation was examined in RAW macrophages at subsaturating levels of protein (5 μg/ml). ApoA1 forms used included isolated human apoA1 (h-ApoA1), recombinant human apoA1 (rh-ApoA1), and the indicated site-directed mutant forms of recombinant human apoA1. 4WF represents recombinant human apoA1 in which the endogenous tryptophans at residues 8, 50, 72, and 108 were converted to phenylalanine. 3MV represents recombinant human apoA1 in which endogenous methionines at residues 86, 112, and 148 were converted to valine. 7YF represents recombinant human apoA1 in which endogenous tyrosines at residues 18, 29, 100, 115, 166, 192, and 236 were converted to phenylalanine. Note that oxidation by the complete MPO system substantially inhibits ABCA1-mediated cholesterol efflux from all apoA1 forms examined except for the oxidant-resistant 4WF mutant. C, recombinant HDL (rHDL) were generated using each of the recombinant human apoA1 forms indicated in B. The capacity of the indicated rHDL to promote BAEC activation in native form versus following oxidation by the MPO/H2O2/Cl− system was then determined by quantifying endothelial cell VCAM-1 surface protein levels. NA refers to “no addition.” Wt refers to rHDL generated with the wild type human sequence for apoA1. D, competition binding data demonstrating that excess MPO-oxidized rHDL generated with the 4WF apoA1 mutant significantly inhibits binding of oxHDL to BAECs. The results represent the means of triplicate determinations of a representative experiment performed at least three times.