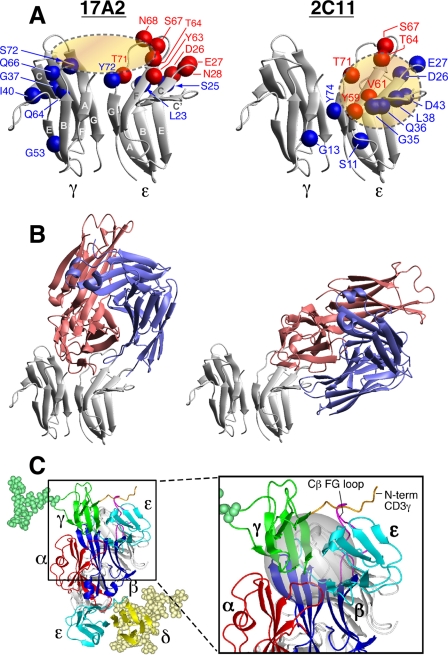
FIGURE 3.
NMR cross-saturation experiments to map antibody binding surfaces on CD3ϵγ and TCR quaternary structure. A, the residues in the binding sites of CD3ϵγ for 17A2 (left) and 2C11 (right) Fabs were mapped using cross-saturation analysis. Blue and red spheres indicate residues that experience significant cross-saturation (signal reduction < 0.5) or disappear upon Fab binding, respectively, and thus mediate direct contact with the Fabs. B, representative models of CD3ϵγ/17A2 Fab and CD3ϵγ/2C11 Fab complexes as described in the text. Red represents the heavy chain fragments and blue the light chain of the Fab. C, proposed TCR quaternary structure model as viewed from the T cell membrane. Magnified boxed region in the complex model shows the location of the Cβ FG loop (magenta) and N-terminal segment of CD3γ (orange) with the Cβ/Cα cave (24) represented as a gray sphere. For simplicity, only CD3γ (green) and CD3δ (yellow) glycans are shown. Hypothetical distance constraints between nearby charged residues were employed with a number of residues, including the entire N-terminal segment of CD3ϵ, which was allowed to be flexible during annealing between the docking steps.