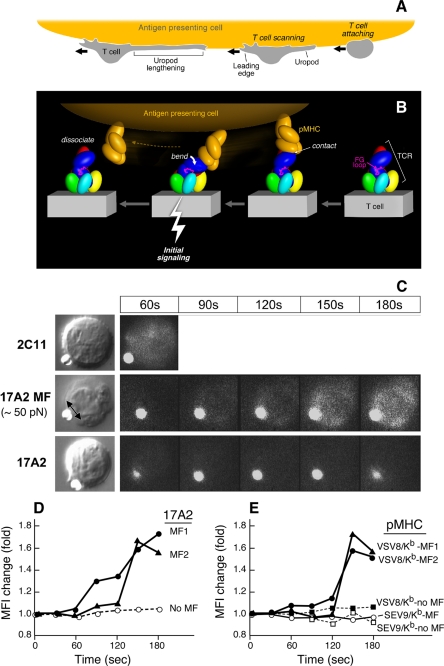
FIGURE 4.
Mechanosensor model for TCR signaling. A, T cell scanning an APC surface in search of specific pMHC. Upon attachment, the T cell assumes a polarized morphology with a leading edge and lengthening uropod. B, one pMHC molecule is shown in orange on the APC, whereas the ectodomains of TCR subunits on the T cell membrane below are colored as described in the legend to Fig. 3. The view is from the CD3ϵγ side. Initial ligation of the TCR by pMHC (right) constitutes a detachable mechanosensor that, as a result of continued T cell scanning, transmits an external torque (bend) into initial signaling (bolt) via the rigid components of the TCR complex prior to dissociation. The β FG loop is shown in magenta. C, calcium flux in naïve T cells after application of external mechanical force using optical tweezers. Equivalent amounts of Alexa 555-labeled 17A2 or 2C11 were immobilized on protein G-coupled polystyrene beads (1 μm diameter). T cell-bead contact was manipulated via the trapping beam as shown in bright field images (left panel). The direction of the external mechanical force (MF) for a 17A2 bead is denoted by the double-headed arrow. Microscopic fluorescence images were recorded using a 532-nm laser for both Alexa 555 and calcium orange (cellular dye for detecting calcium flux) under a temperature control at 37 °C (right panels). For 2C11 beads, the 60-s point only is shown, given the rapidity of calcium flux and the absence of a requirement of any additional MF. The fluorescent antibody-bound bead is the smaller object next to the T cell in every frame. D, quantitative intracellular calcium dynamics upon 17A2 bead interaction. Results are representative of 112 cellular N15 T cell events quantitated in eight separate experiments. Two-tailed t test was performed to compare MFI values of all cells between the 150- and 180-s intervals. Only 17A2 bead with applied tangential mechanical force significantly induces the intracellular calcium flux (p < 0.05). Three representative T cells are shown; two receiving ∼50 pN mechanical force (MF1/2) and one without (No MF). E, calcium dynamics upon pMHC bead interaction. Equivalent numbers (∼1,000) of VSV8/Kb and SEV9/Kb complexes were immobilized on streptavidin-coupled polystyrene beads (1 μm diameter). Tangential mechanical force (∼50 pN) was provided for each of three pMHC beads (pMHC MF) but not two others (no MF). Five representative cells are shown among 190 cell determinations analyzed. Only VSV8/Kb beads upon MF application induced significant calcium flux (p < 0.05) when ∼10 and ∼1,000 pMHC complexes per bead were examined. The extent of calcium flux was not diminished at the lower pMHC complex density nor kinetics of activation lengthened.