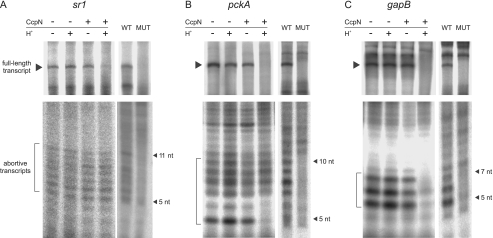
FIGURE 3.
In vitro transcription and detection of abortive transcripts at the sr1 (A), pckA (B), and gapB (C) promoters. Transcription was performed in in vitro transcription buffer (see “Experimental Procedures”) using 100 nm DNA template and 100 nm His-tagged B. subtilis RNA polymerase. 300 nm CcpN-His5 was added, or pH was lowered where indicated. Half of each reaction was separated on either a 6% denaturing polyacrylamide gel to detect the full-length transcripts, indicated by an arrow or on a 23% denaturing polyacrylamide gel to detect abortive transcripts, indicated by a bracket. Control experiments to the right of each panel show which of the abortive transcripts are produced by the investigated promoters. WT, wild-type promoter, MUT, mutated promoter, where the −10 regions have been replaced by the sequence GCCGAT (sr1) or GCCGCT (pckA and gapB). The estimated size of the abortive transcripts on each gel is indicated by arrows. Autoradiograms of the gels are shown.