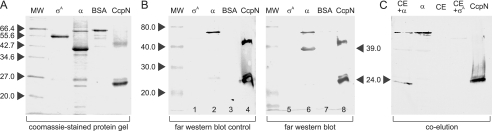
FIGURE 4.
CcpN-RpoA interaction studies. The corresponding molecular weights of the marker bands are indicated beside the marker lanes. A, 15.5% SDS-polyacrylamide gel of different purified proteins. MW, molecular weight marker; σ, purified SigA-His6; α, purified RpoA-His6; BSA, bovine serum albumin; CcpN, purified CcpN-His5. 1 μg of each protein was loaded into each lane. B, Far Western blot of the protein gel shown in A. Equal amounts of protein were loaded into lanes 1–4 and 5–8, respectively. Proteins were renatured after blotting by washing with SDS-free phosphate-buffered saline. Lanes 1–4 are control lanes and were just blocked, incubated with rabbit-anti-CcpN antibody and subsequently with horseradish peroxidase coupled anti-rabbit antibody. Lanes 5–8 are the sample lanes and were treated like lanes 1–5, but were incubated with 200 nm CcpN-His5 after blocking and before incubation with anti-CcpN antibody. The blots were developed using horseradish peroxidase catalyzed conversion of diaminobenzidine. PC-BAS 2.08e software was used for quantification. C, co-elution of RpoA-His6 and CcpN. The lanes were loaded as follows: CE+α: RpoA-His6 preincubated with B. subtilis DB104 protein crude extract (see “Experimental Procedures”) and subsequently purified using a Ni2+-NTA-agarose column; α, RpoA-His6 without preincubation with B. subtilis DB104 protein crude extract; CE, B. subtilis DB104 protein crude extract, purified; CE+σ, SigA-His6 preincubated with B. subtilis DB104 protein crude extract and subsequently purified; CcpN, purified CcpN-His5. Equal amounts of eluate were loaded into each lane.