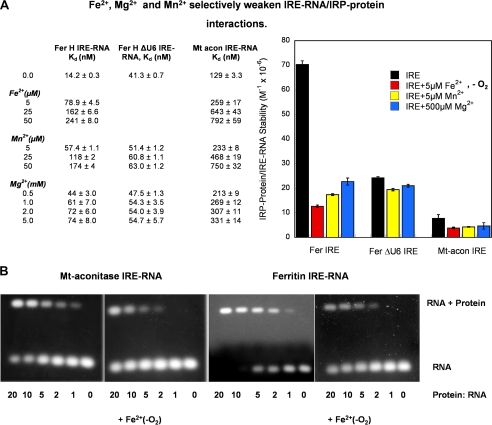
FIGURE 3.
Fe2+ selectively weakens IRE-RNA/IRP1 interactions. A, IRE-RNA binding to IRP was measured, ±Fe2+-O2, as IRP1 fluorescence quenching in solution (constant protein concentration) as described in the legend to Fig. 2 (see “Experimental Procedures”). The IRE-RNAs used were FerH IRE-RNA, FerH ΔU6 IRE-RNA, and mt-aconitase IRE-RNA. Data were analyzed as previously described (45, 46). The Kd values are the averages of three titrations. Relative stabilities (1/Kd) of the IRE-RNA·IRP1 complexes are used in the graph, right. B, IRE-RNA binding to IRP ± Fe2+-O2, was measured by electrophoretic mobility shift assay (constant RNA concentration). Anaerobic solutions of RNA and protein were prepared as in A; Fe2+ was 50 μm when present (see “Experimental Procedures”). A constant 0.1 μm RNA concentration was used with varying concentrations of protein; RNA was stained with ethidium bromide. Note the larger amount of IRP1 repressor required to bind (shift) an equivalent amount of mt-aconitase IRE-RNA compared with ferritin IRE-RNA ± Fe2+-O2. The data are representative of two independent experiments and the significance of the effect of metal ions on the Kd values is p < 0.01.