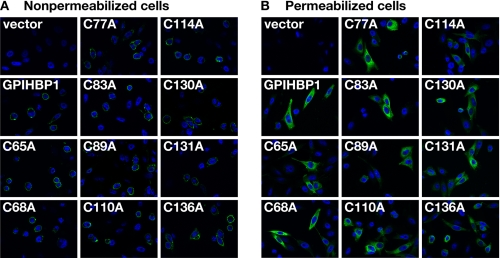
FIGURE 2.
Mutant GPIHBP1 proteins with cysteine-to-alanine mutations within the Ly6 domain reach the cell surface. CHO pgsA-745 cells were electroporated with an empty vector, a construct encoding an S-protein-tagged human GPIHBP1, or mutant GPIHBP1 constructs in which specific cysteines within the Ly6 domain were replaced with alanine. The presence of GPIHBP1 in nonpermeabilized (A) and permeabilized (B) cells was assessed by immunofluorescence microscopy with a goat antiserum against the S-protein tag (green). The cell nuclei were visualized with 4′,6-diamidino-2-phenylindole (blue).