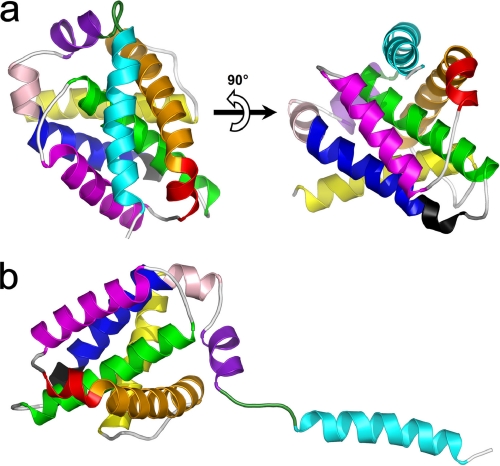
FIGURE 1.
Three-dimensional model of full-length human Bfl-1. a, ribbon representations of Bfl-1 in its compact form with helix α9 placed in the hydrophobic BH3-binding cleft. b, Bfl-1 in its extended form with helix α9 protruding from the globular core. α-helices are colored sequentially from N- to C-terminal ends as follows: yellow, α1 (aa 2–21); orange, α2 (aa 32–51); red, α3 (aa 53–58); purple, α4 (aa 64–79); green, α5 (aa 86–106); blue, α6 (aa 116–130); pink, α7 (aa 132–136); magenta, α8 (aa 139–148); and cyan, α9 (aa 155–173). The 310-helix (aa 113–115) is shown in black. The loop connecting α8 and α9 is shown in dark green (aa 149–154). Orientation of the model in b is deduced from that of a by a 90° clockwise rotation in the plane.