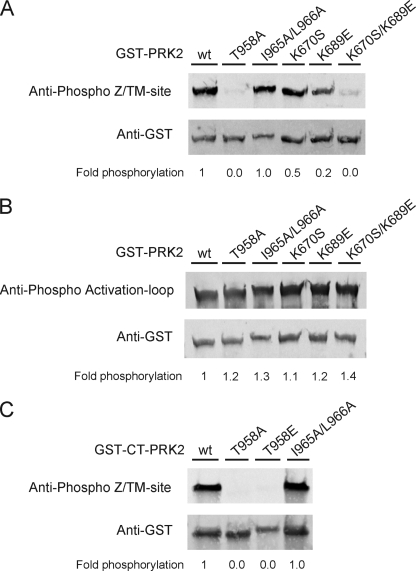
FIGURE 3.
Z/TM site and activation loop phosphorylation of PRK2 constructs employed in this study. HEK293 cells were transfected with DNA constructs encoding full-length PRK2 or the C-terminal region (CT) of PRK2 as GST fusion proteins. The cells were lysed 48-h post-transfection, and the GST fusion proteins were purified with glutathione-Sepharose. Bound proteins were eluted with SDS-sample buffer (A and B) or glutathione (C). Aliquots of the proteins were subjected to SDS-PAGE followed by immunoblotting. Phosphorylation of the Z/TM-site (A and C) and the activation loop site (B) was detected with phosphospecific antibodies for the respective site. Total protein amounts were detected using an anti-GST antibody. Secondary antibodies were fluorescently labeled and immunoblots were developed with a FLA-9000 Starion (Fujifilm). The extent of phosphorylation was quantified using the program MultiGauge V3.0 (Fujifilm) and normalized over the amount of loaded protein. A value of 1 was assigned to the phosphorylation of wt GST-PRK2 (A and B) or wt GST-CT-PRK2 (C).