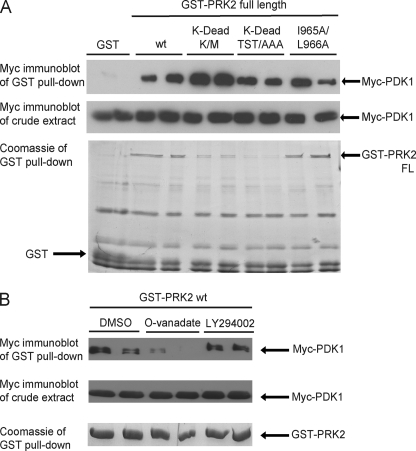
FIGURE 5.
Mutations that abolish the activity of PRK2 increase its interaction with PDK1. HEK293 cells were transfected with DNA constructs expressing Myc-PDK1 together with constructs expressing GST or GST-PRK2 fusion proteins. The interaction was analyzed as described in the legend to Fig. 2. Duplicates of each condition are shown. The extent of PDK1 binding was quantified using the program MultiGauge V3.0 (Fujifilm) and normalized over the amount of immobilized PRK2. A value of 1 was assigned to wild-type PRK2. A, mutation of Lys-686 in the ATP-binding site to Met (PRK2[K-dead K/M]) or substitution of Thr-814, Ser-815, and Thr-816 in the activation loop for Ala (PRK2[K-dead TST/AAA]) generated inactive, so-called kinase-dead (K-dead), PRK2 proteins. These proteins were expressed in HEK293 cells to a lower level than wild-type PRK2 (wt). However, the interaction between PDK1 and PRK2[K-dead TST/AAA] or PRK2[K-dead K/M] was even increased compared with PRK2 wt (3.7-fold for both K-dead PRK2 constructs). Mutation of Ile-965 and Leu-966 to Ala (I965A/L966A) in full-length PRK2 had no effect on the interaction with PDK1 (1.1-fold binding). B, GST-PRK2 wt was co-expressed with Myc-PDK1, and the cells were treated with 1 mm orthovanadate (O-vanadate), 50 μm LY294002, or DMSO as a control 2 h before cell lysis. The treatment with LY294002 had no effect on the interaction between PRK2 and PDK1 while the addition of orthovanadate led to a decreased binding of PRK2 to PDK1.